ACE-2 and Immune System Changes in Smokers May Underlie COVID-19 Vulnerability
Clinicians report that people with pre-existing conditions such as cardiac disease, hypertension, and diabetes are at higher risk of mortality from COVID-19. With tobacco smoking being the leading cause of preventable death worldwide, it is surprising that smokers are underrepresented in hospital records1. While hospital record data gives insight into the risk factors that influence COVID-19 outcomes, tobacco studies provide a further understanding of how smoking compromises the immune system. In fact, many human and rodent studies show that smoking increases the expression of angiotensin-converting enzyme 2, also known as the ACE-2 receptor and entry point for the SARS-CoV-2 virus2. Normally, ACE-2 has a protective role in the cardiovascular system by regulating vasoconstriction, inflammation, and tissue damage. These protective functions are inhibited once the SARS-CoV-2 virus binds to ACE-2, and receptors levels then decrease following infection3, thereby allowing disease-causing biochemical processes to develop. ACE-2 levels are also known to vary among individuals, and people with cardiopulmonary diseases and those who take medications that help lower blood pressure also have high expression of the receptor 4. Therefore, human and animal studies focusing on the role of ACE-2 in cardiopulmonary disease and immune defenses provide insight that may be helpful for establishing biomarkers of COVID-19 disease progression and developing medication strategies for susceptible populations
Recent studies provide evidence that both traditional cigarettes and electronic cigarette (eCig) devices alter ACE-2 activity. One study assessed the levels of ACE-2 and transmembrane serine protease-2, which also facilitates viral entry, in peripheral blood mononuclear cell samples of young smokers collected before the pandemic2. These young smokers had elevated levels of ACE-2 compared to non-smokers, and the effects were stronger in traditional cigarette smokers than in eCig users. Interestingly, the plasma cotinine levels (a measure of tobacco smoke exposure) were comparable between cigarette and eCig smokers, suggesting that non-nicotine components of traditional cigarettes may play a significant role in altering the immune system. While the study demonstrates that changes in ACE-2 could potentially increase susceptibility to viral entry and promote COVID-19 complications even among young healthy smokers, this study does not suggest that eCigs can be used as an effective harm-reduction strategy.
Animal models allow researchers to study biological effects in a controlled environment, in which animals are exposed to identical conditions. Studies using rodent models also confirm that the molecular players involved in SARS-CoV-2 infection are modulated by smoking. In one study, mice were exposed to eCigs with and without nicotine for 21 days and developed airway inflammation and immune cell infiltration in the lung5. Interestingly, ACE-2 protein levels were also increased in eCig exposed animals, but the effect was stronger in male mice as compared to female mice. There was also a greater effect as male mice exposed to eCig vapor and co-exposed to nicotine, which suggests that changes in ACE-2 protein are influenced by nicotine in a dose-dependent manner and sex-based differences may also be relevant to infection. While replicating such a study in humans to determine whether smoking directly influences viral entry may not be realistic, rodent studies provide valuable insights into sex-specific effects in animals exposed to controlled levels of toxicants.
While it is well established that smoking can promote immune dysregulation and COVID-19 complications, many questions remain as to how nicotine dosage, non-nicotine components, and pollutants unique to eCig devices also influence health outcomes. Processes like genetic heterogeneity of human populations and human expression of proteins that promote viral entry may also underlie susceptibility to COVID-19 mortality, which remains an exciting area of research. Scientific efforts across many fields of discipline continue to uncover the relationship between smoking and ACE-2, and novel findings continue to inform developing clinical trials to study the efficacy of medication for COVID-19 among smokers and patients with cardiopulmonary diseases.
References.
- Monterrosa Mena, J. Insights About COVID-19 Health Outcomes in Smokers from Hospital Records, https://earlycareervoice.professional.heart.org/insights-about-covid-19-health-outcomes-in-smokers-from-hospital-records/
- Kelesidis, T., Zhang, Y., Tran, E., Sosa, G., & Middlekauff, H. R. (2021). Instigators of COVID-19 in Immune Cells Are Increased in Tobacco Cigarette Smokers and Electronic Cigarette Vapers Compared With Nonsmokers. Nicotine & Tobacco Research, ntab168. https://doi.org/10.1093/ntr/ntab168
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005 Aug;11(8):875-9. doi: 10.1038/nm1267.
- Igase, M., Kohara, K., Nagai, T. et al. Increased Expression of Angiotensin Converting Enzyme 2 in Conjunction with Reduction of Neointima by Angiotensin II Type 1 Receptor Blockade. Hypertens Res 31, 553–559 (2008). https://doi.org/10.1291/hypres.31.553
- Naidu, V., Zeki, A. A., & Sharma, P. (2021). Sex differences in the induction of angiotensin converting enzyme 2 (ACE-2) in mouse lungs after e-cigarette vapor exposure and its relevance to COVID-19. Journal of Investigative Medicine, 69(5), 954–961. https://doi.org/10.1136/jim-2020-001768
“The views, opinions, and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness, and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions, or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your health matters. If you think you are having a heart attack, stroke, or another emergency, please call 911 immediately.”


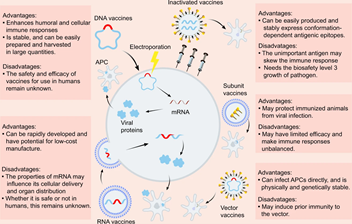
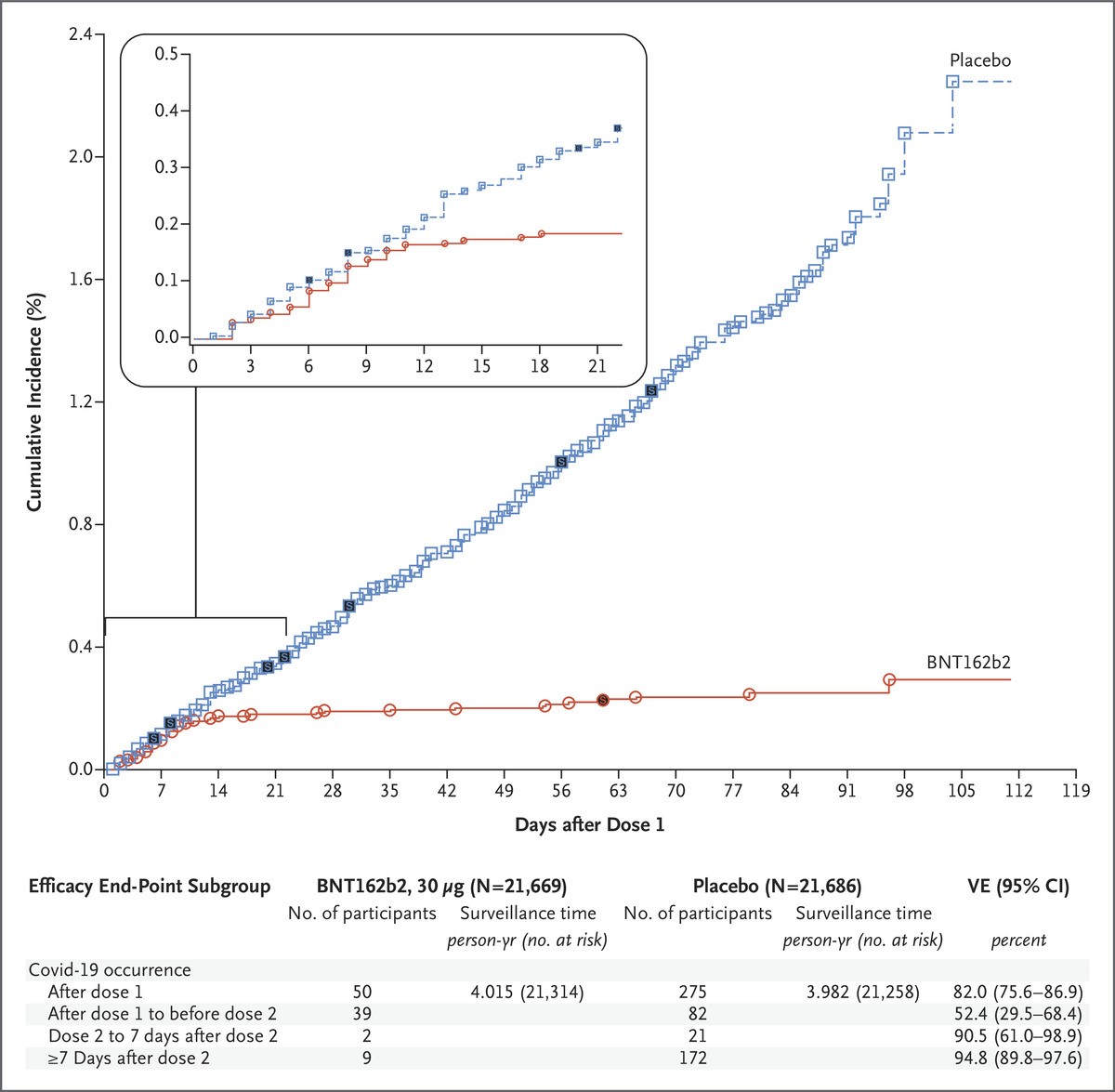
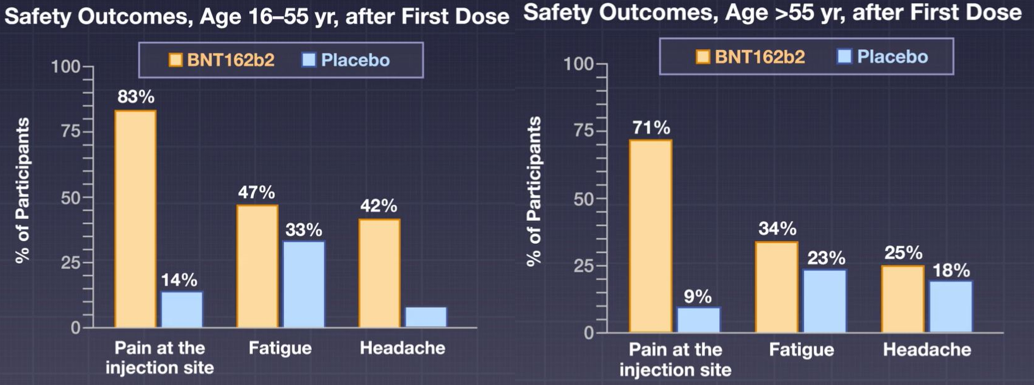
 When news of the approval of the Pfizer vaccine surfaced, I felt a sense of anxiety. There was no data on pregnant women in the COVID-19 vaccine trials. As a medical professional, we have been trained to apply for evidenced-based medicine and baseline patient characteristics of trial participants to the patients we plan to treat. But what if your pregnancy status was not studied in the trial during a global pandemic? How might you weigh the unknown risks of the vaccine to your growing fetus with the risk of COVID-19 infection while pregnant?
When news of the approval of the Pfizer vaccine surfaced, I felt a sense of anxiety. There was no data on pregnant women in the COVID-19 vaccine trials. As a medical professional, we have been trained to apply for evidenced-based medicine and baseline patient characteristics of trial participants to the patients we plan to treat. But what if your pregnancy status was not studied in the trial during a global pandemic? How might you weigh the unknown risks of the vaccine to your growing fetus with the risk of COVID-19 infection while pregnant?