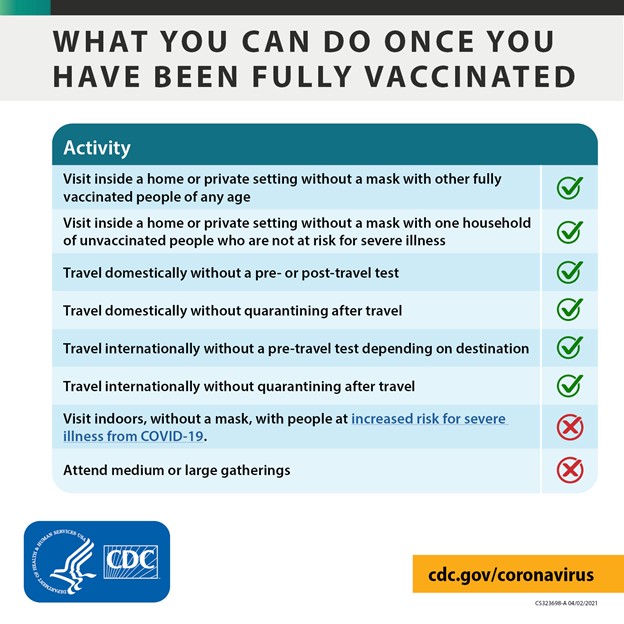
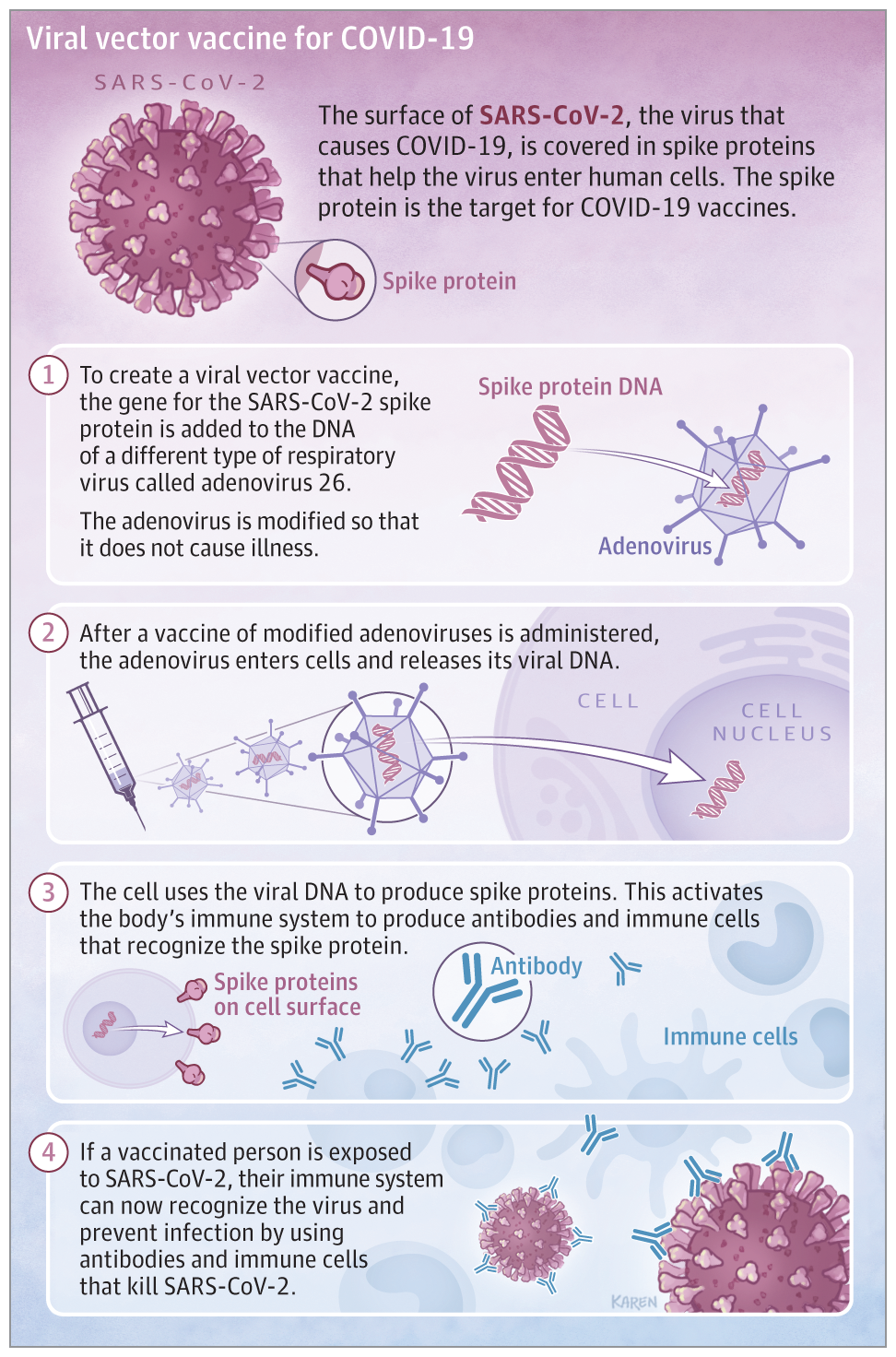
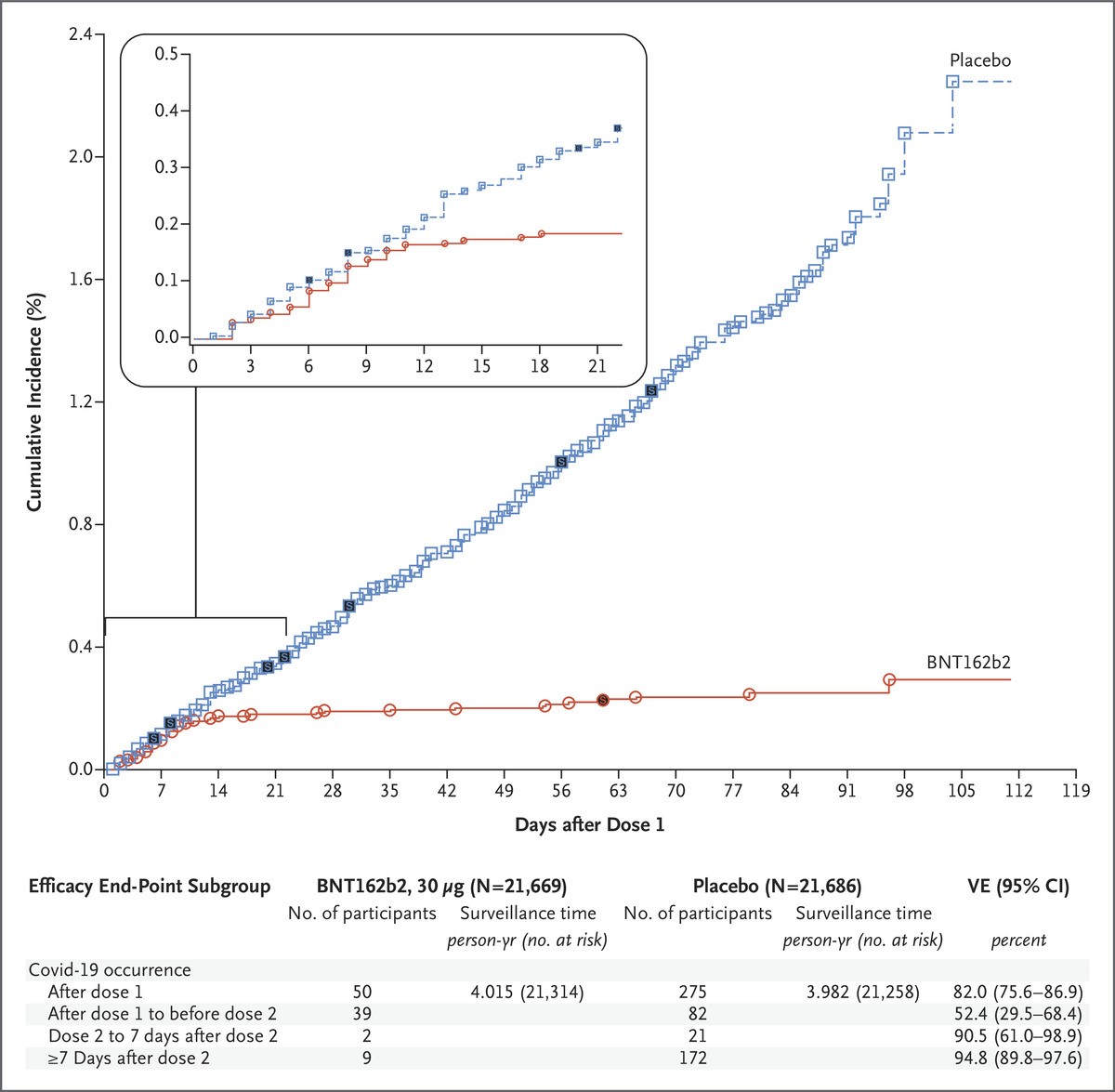
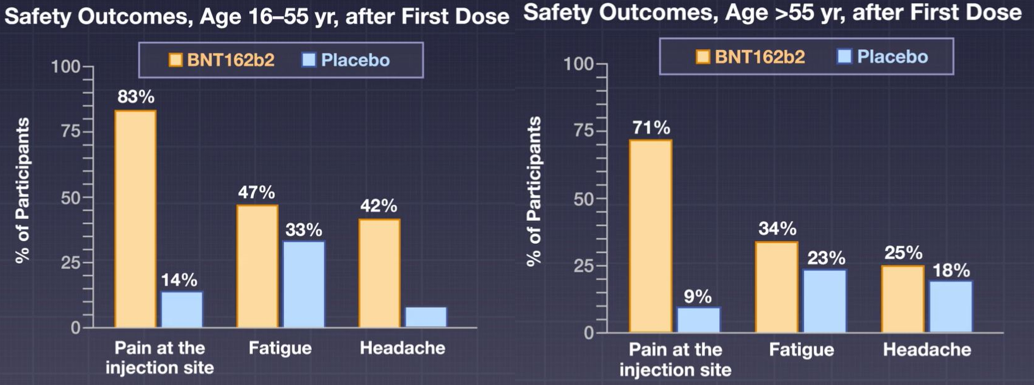
Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock
Cardiogenic shock (CS) is caused by severe impairment of myocardial performance causing a lack of end-organ perfusion. CS carries a very high mortality and in the past few decades, the only intervention that provided clear survival benefits was early revascularization. In the late 1990s, the widespread availably of percutaneous coronary interventions led to an improvement in the CS mortality rate. However, afterward, the mortality rate plateaued despite all the new developments in mechanical support devices. Recently the American Heart Association (AHA) published a scientific statement to guide managing patients presenting with myocardial infarction complicated by cardiogenic shock. (1) In this blog, I will review the document’s highlights.
Key points:
– The lack of a standardized cardiogenic shock definition led to uncertainty in comparison of outcomes across the nation.
– Endorsement of the Society for Cardiovascular Angiography and Intervention (SCAI) new classification schema for cardiogenic shock. (Figure 1) (2) Based on the SCAI classification, the AHA statement proposed their management guidelines. (Figure 2)

Figure 1: SCAI’s classification of cardiogenic shock. ( adapted from Baran DA et al.)(2)
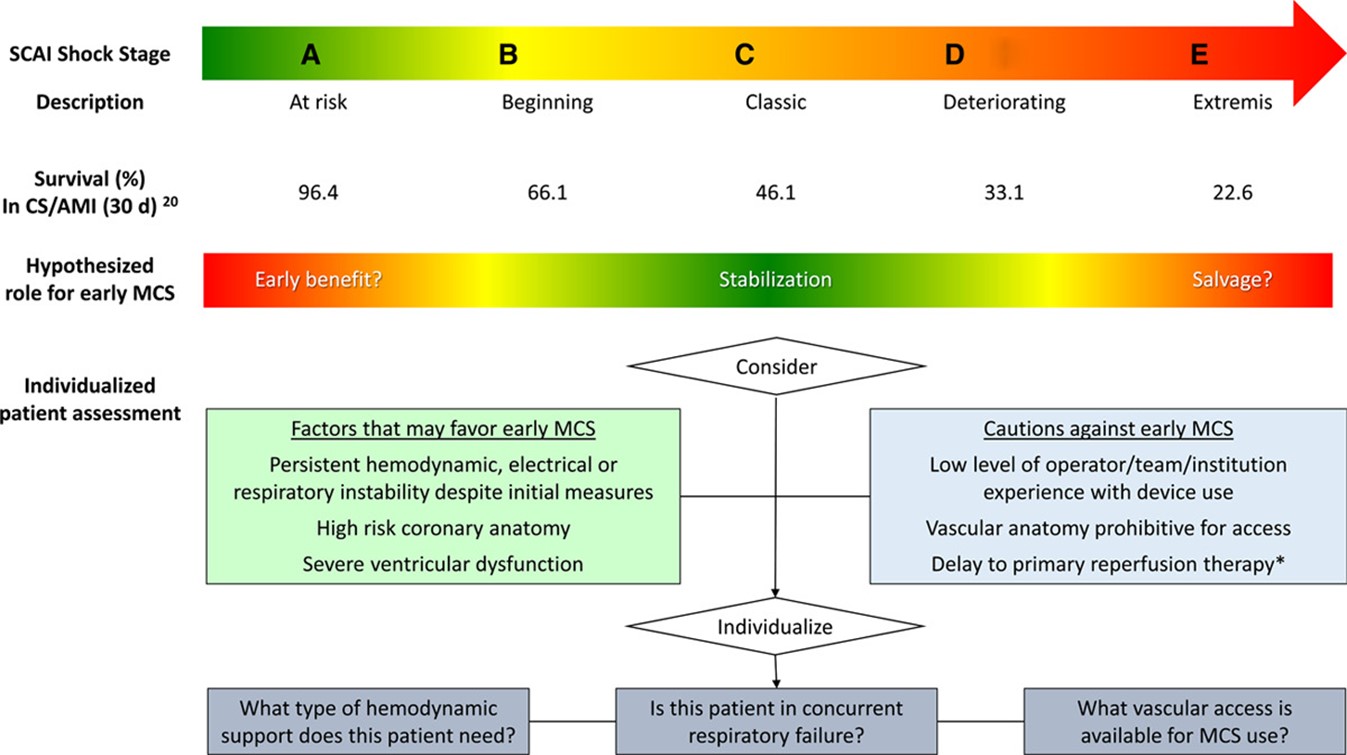
Figure 2: Consideration of early mechanical circulatory support (MCS) in the context of shock classification. (Adapted from Henry TD et al.)(1)
– Use the minimum necessary dose of vasopressors to maintain a mean arterial blood pressure of 65 and above.
– Norepinephrine is your first go-to pressor.
– In unstable bradycardia, Epinephrine or dopamine is recommended.
– In dynamic LVOT Obstruction, use pure vasopressors such as phenylephrine or vasopressin.
– In refractory hypoxemia or severe acidosis, the efficacy of catecholamines is compromised, hence vasopressin is recommended.
– Worsening hypoxemia or severe acidosis increase the risk of ventricular fibrillation and death, hence early endotracheal intubation and mechanical ventilation is recommended.
– Echocardiogram should be performed as soon as possible with focusing on left and right ventricular function, valvular lesions, pericardial effusion/tamponade, and mechanical complications.
– Patients who are relatively stable (stage A and B) should be brought to the cardiac catheterization lab as soon as possible. However, patients in stages C, D, and E should be stabilized first with minimal delay.
– PCI of the culprit’s vessel is recommended regardless of the delay. In cases of multivessel disease, PCI should be performed on the culprit lesion only. Prior to giving contrast, LVEDP should be documented if possible.
– Given that CS is a risk factor for stent thrombosis. Third-generation oral PY12 is recommended over clopidogrel. However, bleeding risk should be evaluated especially in the setting of large-caliber access for MCS.
– RHC is not required to diagnose CS especially if it will delay reperfusion. However, invasive measurements could guide management. There are no randomized clinical trials to validate its routine use.
– Over the past decade, several MCS devices were developed. Although theoretically, MCS should help patients with CS, so far, the data behind it is very limited.
– MCS should be considered in patients who are persistently hypoperfused and hypotensive on vasopressors with low cardiac index.
– For patients with Left ventricular failure, Intra-aortic balloon pump (IABP), Impella, Tandem heart or VA ECMO should be considered. In right ventricular failure, consider Impella RP or Protek Duo. In patients with Biventricular failure, consider Bilateral Impella pumps or VA-ECMO with a venting device (IABP or Impella). (Figure 3)
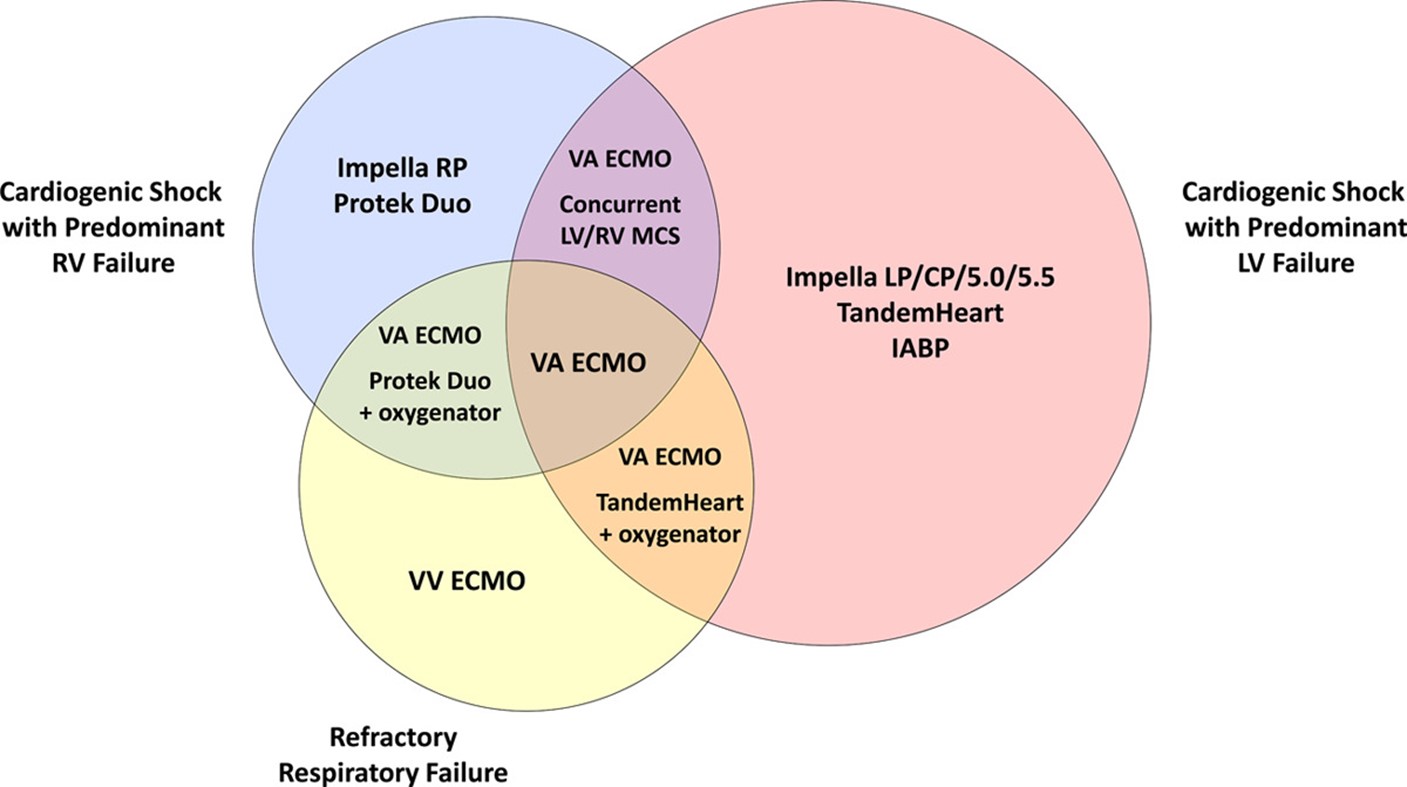
Figure 3: Mechanical support devices suggested according to the clinical picture. (Adapted from Henry TD et al.)(1)
CS continues to be a very complex entity with very high mortality. The difficulty in conducting trials in this vulnerable population is one of the main challenges. In order to fill this gap, the AHA statement outlined the essential areas for future research.(1) Multiple studies are being conducted and hopefully, these studies will provide us with valuable information to improve the outcomes of this morbid condition.
References:
- Henry TD, Tomey MI, Tamis-Holland JE, et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2021;143(15):e815-e29.
- Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94(1):29-37.
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”