Yes, we are still in the middle of the COVID pandemic. With the help of more people getting vaccinated and mask mandates in effect, a post-pandemic world is no longer a mere imagination. While waiting for the pandemic to be over, there are some doubts about whether the COVID vaccines should be cleared to facilitate a faster transition back to normal life.
- What are the leading COVID vaccines?
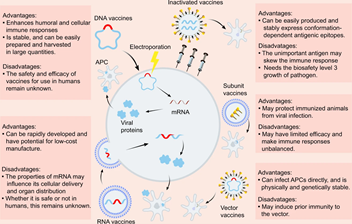
Figure: Overview of the diverse types of vaccines, and their potential advantages and disadvantages (Dong et al. 2020).
Currently, two COVID-19 vaccines are authorized and then recommended for use in the United States–the Pfizer-BioNTech COVID-19 vaccine(Polack et al. 2020) and the Moderna’s COVID-19 vaccine(Baden et al. 2020). Both of the vaccines used a cutting-edge technology, the messenger RNA (mRNA) vaccine which has been developed in the 1990s.
As of December 28th, 2020, three other COVID-19 vaccines are undergoing large-scale (Phase 3) clinical trials in the United States: AstraZeneca’s COVID-19 vaccine(Knoll and Wonodi 2021), Janssen’s COVID-19 vaccine and Novavax’s COVID-19 vaccine(Sadoff et al. 2021). Both the AstraZeneca COVID-19 vaccine and Janssen’s COVID-19 vaccines (Johnson& Johnson) used a weakened adenovirus vector strategy to tackle the spike protein on the SARS-CoV-2 virus. The weakened virus vector serves as a “Trojan horse” to deliver “information” to the cells in order to stimulate the memory of immune defense against SARS-CoV-2 virus. The adenovirus-based vaccines are relatively less foreign to the public, currently they are used against a wide variety of pathogens such as Mycobacterium tuberculosis, human immunodeficiency virus (HIV), and Plasmodium falciparum. The AstraZeneca COVID-19 vaccine has already authorized to use in Europe on January 12th, 2021 and possibly obtains approval in the United States early 2021. On January 29th, Johnson& Johnson announced its interim clinical Phase 3 trial results and a single-shot Janssen COVID-19 vaccine is on the way for FDA approval.
Novavax COVID-19 vaccine, a protein subunit-based vaccine, just announced its interim UK Phase 3 clinical trial results on January 28th, 2021. It shows promising protection to the SARS-CoV-2 virus, as well as the UK and South Africa variants. The company has already signed purchase agreements with many governments including Australia and Canada.
Two other vaccines– Russia’s sputnik V vaccine and China’s COVID-19 vaccine developed by Sinovac Biotech are also the lead runners in the vaccine race. The sputnik V vaccine which has obtained authorization to use in Russia back in November 2020, just published its Phase 3 data on February 2nd(Logunov et al. 2021). It’s an adenovirus-based vaccine, similar as the AstraZeneca COVID-19 vaccine and Janssen’s COVID-19 vaccine.
China’s COVID vaccine used a relatively well-understood technology: an inactivated SARS-CoV-2 virus. The inactivated virus vaccine approach has been implemented for a wide range of vaccines such as polio vaccine, hepatitis A vaccine, rabies vaccine and most flu vaccines. So far it received some inconsistent results from Brazil, Indonesia and Turkey and it’s not applicable in the United States. Overall, the efficacy is encouraging (50.38% to 91.25%) and requires more data to reach a more consistent result.
- How to understand the efficacy?
It’s a numbers game or is it? The high efficacy (95%) data released from Pfizer and Moderna at the end of last year received with great applause. The 70% protection starting after a first dose from AstraZeneca seems less impressive. The AstraZeneca COVID-19 vaccine confirms 100% protection against severe disease, hospitalization and death in the primary analysis of Phase 3 trial suggesting a total success. The recent Phase 3 trial results from Johnson& Johnson’s single-shot vaccine shows 72% effective in the United States and 66% effective overall at preventing moderate to severe COVID-19, 28 days after vaccination. The efficacy number simply cannot be interpreted as the higher the better. Like all of the clinical trials, compounding factors need to take into consideration. Their vaccine impact may depend on sex, age, genetics, geography, the timing of assessment of the end-point, the percentage of population affected by new variant compared to the original variant.
The thing matters the most is to reduce hospitalization and death. So far most of the leading vaccines have showed great promise. At the current stage, whatever vaccine is available to you could protect you from getting serious disease and prevent the virus spread to your loved ones one way or another. Herd immunity could finally be reached if enough people are getting vaccinated in the near future.
- mRNA technology: what is it? And is it safe?
Considering mRNA vaccine is the new kid on the block, it’s understandable that certain hesitancy and reluctance towards getting vaccinated. mRNA therapy has been developed and used to target certain types of cancer for more than twenty years. It has recently been used to target SARS-CoV-2 virus. The nucleic acid fragment of SARS-CoV-2 virus spike protein is packaged in a lipid nanoparticle. Like how most vaccine works, it tricks your body to formulate a defense memory using a small piece of information from the virus. When the actual attacks occurred, you are protected with a pre-programmed defense mechanism already. It does not change your DNA. It just helps your body to remember what it feels like to successfully combat the virus. Some of the side effects from clinical trials could be another reason to cause hesitancy. Don’t blame the messenger. The individual response elicited by the vaccines is just a small fraction of what you might experience when the real attack occurs. Some extreme allergic responses, a few reported in a million cases are rare. The chance is as similar as winning a Powerball or Mega Millions lottery. At the end of the day, the benefits still outweigh the risks.
- Early progress and new variants
Israel’s vaccination program shows encouraging outcome, results from a recently published preprint(Chodick et al. 2021). It’s in agreement with the Phase 3 clinical trial results from Pfizer. Data collected by Israel’s Ministry of Health shows a 41% reduction in confirmed COVID-19 infections in people aged 60 and order. Close to 90% of that age group has been administered with the first dose of Pfizer’s 2-dose vaccine. For people aged 59 and younger, the infected cases and hospitalization are also dropped.
Viruses like SARS-CoV-2 mutate all the time. There are 3 concerned variants: the UK variant (B.1.1.7), Brazil (P.1) and South Africa (B.1.351) have already been found in the United States. With the surge of new variants of SARS-CoV-2, the effectiveness of the COVID-19 vaccine also dropped. Some new data from Johnson& Johnson and Novavax suggest that the COVID-19 vaccines can prevent a lot of mild and moderate cases, and are still very effective against preventing hospitalization and deaths. Other company such as Moderna, has already developed booster shots to combat new variants. If most of the population got vaccinated, it will stop the virus’s replication and ultimately stop mutation completely. The recommended measure is to vaccine as many people as possible at current stage.
In conclusion, no matter which vaccine you got or are going to get, as long as it’s approved and authorized by the FDA, the chance of having effective protection is still very good. At the end of the day, the benefits outweigh the risks.
Reference
Baden, Lindsey R., Hana M. El Sahly, Brandon Essink, Karen Kotloff, Sharon Frey, Rick Novak, David Diemert, et al. 2020. “Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine.” New England Journal of Medicine. https://doi.org/10.1056/nejmoa2035389.
Chodick, Gabriel, Lilac Tene, Tal Patalon, Sivan Gazit, Amir Ben Tov, Dani Cohen, and Khitam Muhsen. 2021. “The Effectiveness of the First Dose of BNT162b2 Vaccine in Reducing SARS-CoV-2 Infection 13-24 Days after Immunization: Real-World Evidence.” MedRxiv, January, 2021.01.27.21250612. https://doi.org/10.1101/2021.01.27.21250612.
Dong, Yetian, Tong Dai, Yujun Wei, Long Zhang, Min Zheng, and Fangfang Zhou. 2020. “A Systematic Review of SARS-CoV-2 Vaccine Candidates.” Signal Transduction and Targeted Therapy. https://doi.org/10.1038/s41392-020-00352-y.
Knoll, Maria Deloria, and Chizoba Wonodi. 2021. “Oxford–AstraZeneca COVID-19 Vaccine Efficacy.” The Lancet. https://doi.org/10.1016/S0140-6736(20)32623-4.
Logunov, Denis Y, Inna V Dolzhikova, Dmitry V Shcheblyakov, Amir I Tukhvatulin, Olga V Zubkova, Alina S Dzharullaeva, Anna V Kovyrshina, et al. 2021. “Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia.” The Lancet, February. https://doi.org/10.1016/S0140-6736(21)00234-8.
Polack, Fernando P., Stephen J. Thomas, Nicholas Kitchin, Judith Absalon, Alejandra Gurtman, Stephen Lockhart, John L. Perez, et al. 2020. “Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine.” New England Journal of Medicine. https://doi.org/10.1056/nejmoa2034577.
Sadoff, Jerald, Mathieu Le Gars, Georgi Shukarev, Dirk Heerwegh, Carla Truyers, Anne M. de Groot, Jeroen Stoop, et al. 2021. “Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine.” New England Journal of Medicine. https://doi.org/10.1056/nejmoa2034201.
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”

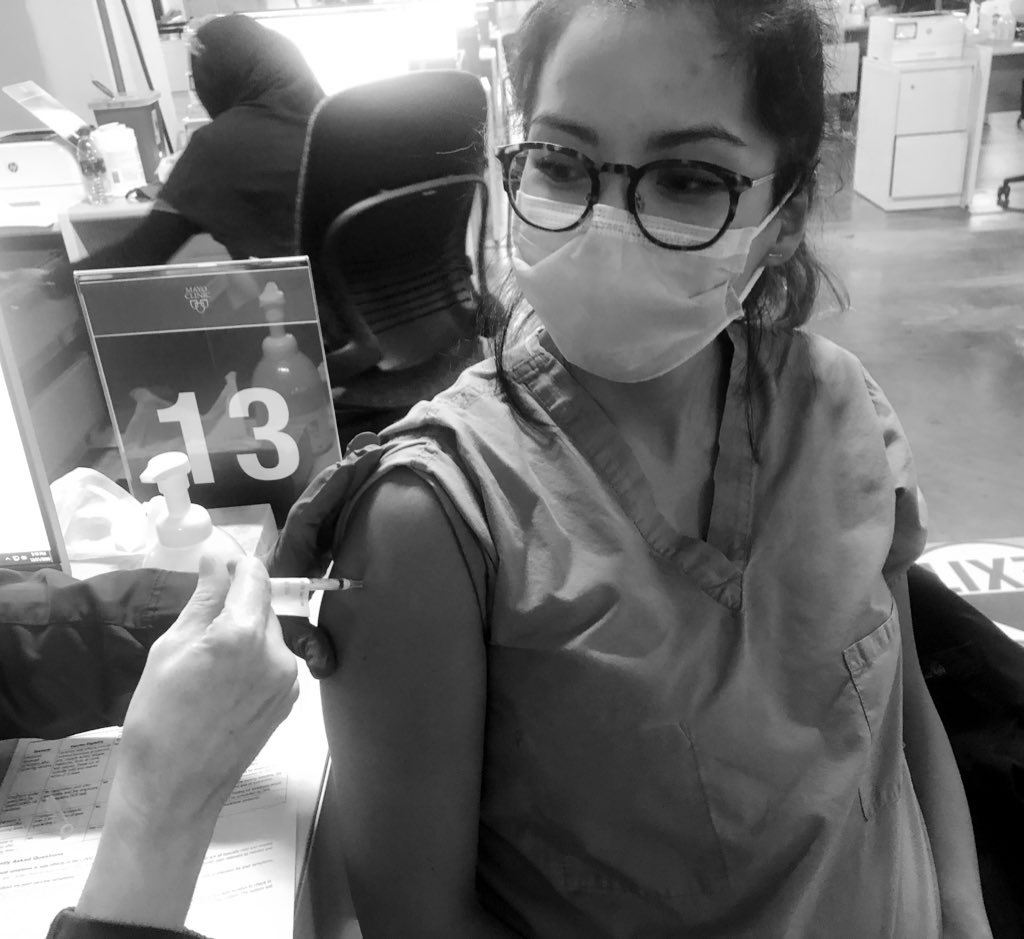 When news of the approval of the Pfizer vaccine surfaced, I felt a sense of anxiety. There was no data on pregnant women in the COVID-19 vaccine trials. As a medical professional, we have been trained to apply for evidenced-based medicine and baseline patient characteristics of trial participants to the patients we plan to treat. But what if your pregnancy status was not studied in the trial during a global pandemic? How might you weigh the unknown risks of the vaccine to your growing fetus with the risk of COVID-19 infection while pregnant?
When news of the approval of the Pfizer vaccine surfaced, I felt a sense of anxiety. There was no data on pregnant women in the COVID-19 vaccine trials. As a medical professional, we have been trained to apply for evidenced-based medicine and baseline patient characteristics of trial participants to the patients we plan to treat. But what if your pregnancy status was not studied in the trial during a global pandemic? How might you weigh the unknown risks of the vaccine to your growing fetus with the risk of COVID-19 infection while pregnant?