Who Am I? Ruminations of a Cardiology Fellow
I walked out of the hospital after my last inpatient shift of my first year of Cardiology fellowship and let out a sigh. It felt like I was releasing a breath I’d been holding in for years. Four years ago, as I started intern year, I told myself that I would dedicate the first years of my career to becoming first the best internist, and then the best cardiologist that I could be. This decision was borne out of a desire to fully invest in my training, but also to some extent out of imposter syndrome. I worried (as many new interns do) about not being smart enough or good enough, so my natural reaction was to work hard to become a better physician.
The ensuing four years, during which I immersed myself in clinical medicine, have been transformative. My experiences as a budding internist and cardiologist reaffirmed time and time again that I chose the right path for me. Though I cannot say that my initial insecurities totally went away, somewhere along the way, I learned to accept and set aside feelings of inadequacy so that I could do my job and take care of my patients. My patients have been my guiding light and have taught me everything I know. Moreover, as a first year Cardiology fellow, I spent countless hours learning the amazing intricacies of cardiovascular pathophysiology. While I have much left to learn in the remainder of my fellowship, I feel much more adept at managing typical cardiovascular problems.
However, these incredible experiences have also come at a personal cost. I have missed weddings, birthdays, funerals of loved ones. I have relinquished hobbies that I used to cherish so that I could prioritize self care, sleep or precious moments with friends and family. Some days I can’t help but wonder if I lost a little bit of myself in devoting myself so wholly in caring for others. Have I sanded away the aspects of my personality that made me unique in service of my training? Who am I, beyond my job as a physician?
Now, don’t get me wrong – I love my job. There’s nothing I’d rather be than a cardiologist. I love being in the echo lab or the cath lab. The cardiac intensive care unit is my happy place. Being in the hospital feels like being at home. But my job also can’t be all that I am.
In my favorite AHA Early Career Voice blog post, Dr. Nasrien Ibrahim wrote about how important it is to “bring your whole self to work.” I loved the piece so much that it inspired me to apply to be a blogger for the AHA Early Career Blogger program myself. I loved the concept that we should bring all parts of ourselves with us in our daily life. “The authentic you,” as Dr. Ibrahim called it. Now, reading that piece back two years later, I ask myself – what parts of myself do I bring with me? What parts have I left behind? What other parts of myself do I need to cultivate and nurture again?
When I move on to my second year of Cardiology fellowship, I hope to have more time to answer these questions and rediscover who I am outside of my day job. It is a given that I will continue to improve my skills as a cardiologist. But I also hope to continue current hobbies, reignite old passions, and maybe even discover some new ones. I hope to write more. I hope to travel more. I hope to spend more time with my loved ones. Only then can I bring “my whole self” with me every day.
To every rising Cardiology fellow reading this, I say: The ride will be wild, but enjoy it as much as you can. Never forget why you decided to become a cardiologist in the first place, especially when you feel overwhelmed by all the demands being placed on your time or the seemingly endless consults you are seeing. Give yourself a chance to do the things that make you happy when you are not at work, but also give yourself some grace and recognize that it can also be ok to do nothing if that is what you need to recharge your battery. When in doubt, look to your patient and ask yourself what you would want from your cardiologist if you were in their shoes. And when you have the time, space and mental bandwidth, ask yourself: who am I and how do I ensure that I preserve myself in this journey?
“The views, opinions, and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness, and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions, or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your health matters. If you think you are having a heart attack, stroke, or another emergency, please call 911 immediately.”
 Blood pressure is the force that blood applies to the walls of arteries as it’s pumped throughout the body.
Blood pressure is the force that blood applies to the walls of arteries as it’s pumped throughout the body.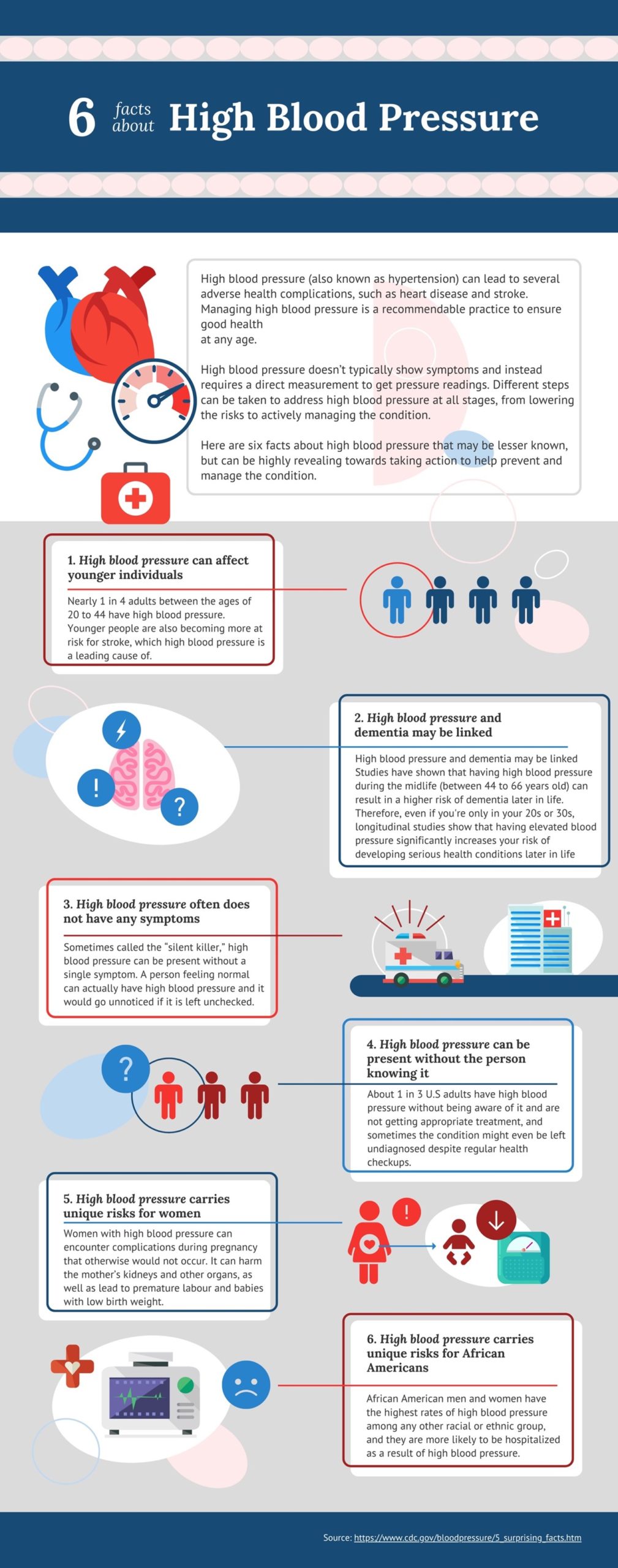
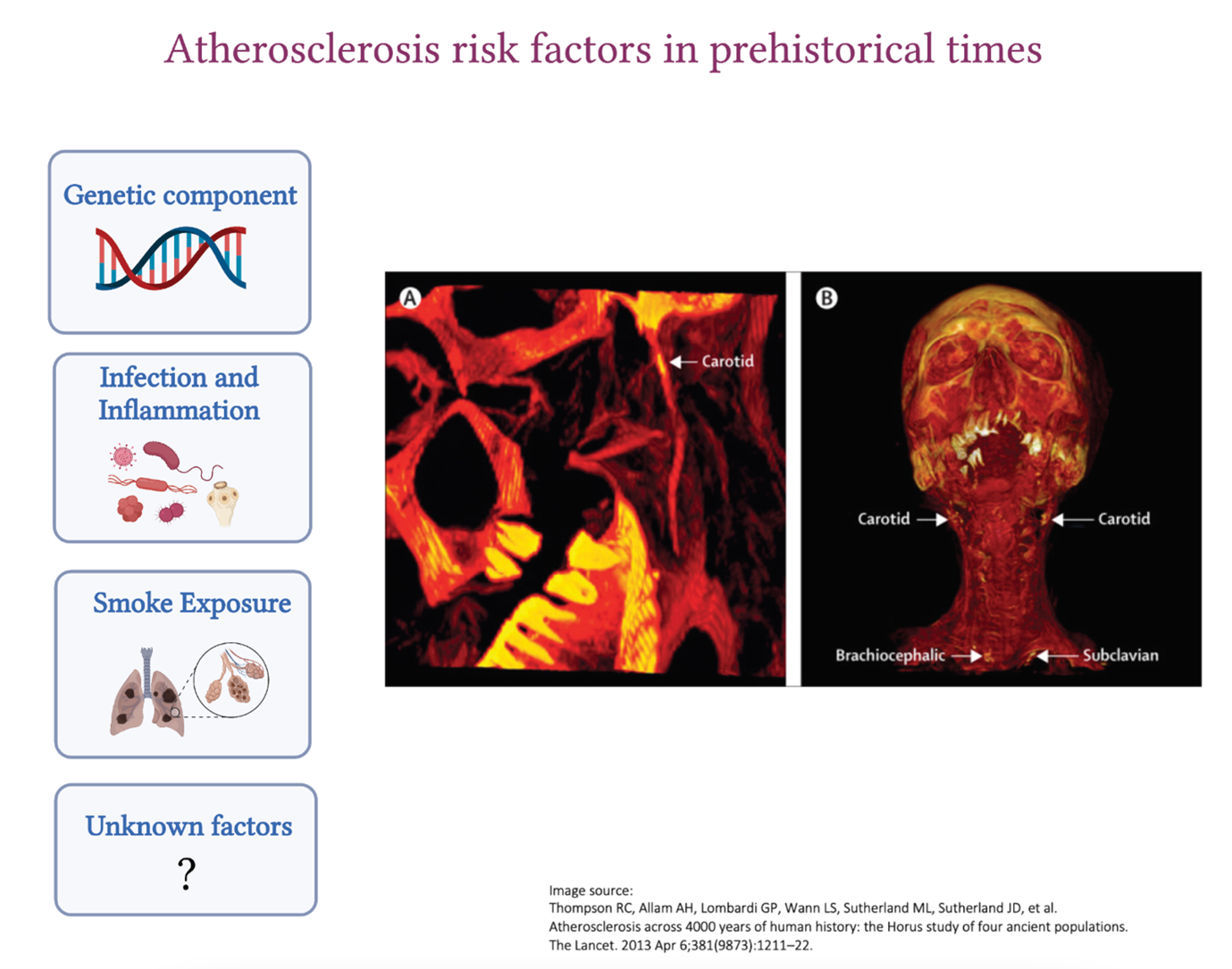

 You might want to check out Vascular Discovery 2022, a 2 ½-day meeting, which is sponsored by the Council on Arteriosclerosis, Thrombosis and Vascular Biology, the Peripheral Vascular Disease Council, and the Council on Genomic and Precision Medicine, in cooperation with and the Society for Vascular Surgery’s Vascular Research Initiatives Conference (Flyer on the right).
You might want to check out Vascular Discovery 2022, a 2 ½-day meeting, which is sponsored by the Council on Arteriosclerosis, Thrombosis and Vascular Biology, the Peripheral Vascular Disease Council, and the Council on Genomic and Precision Medicine, in cooperation with and the Society for Vascular Surgery’s Vascular Research Initiatives Conference (Flyer on the right).