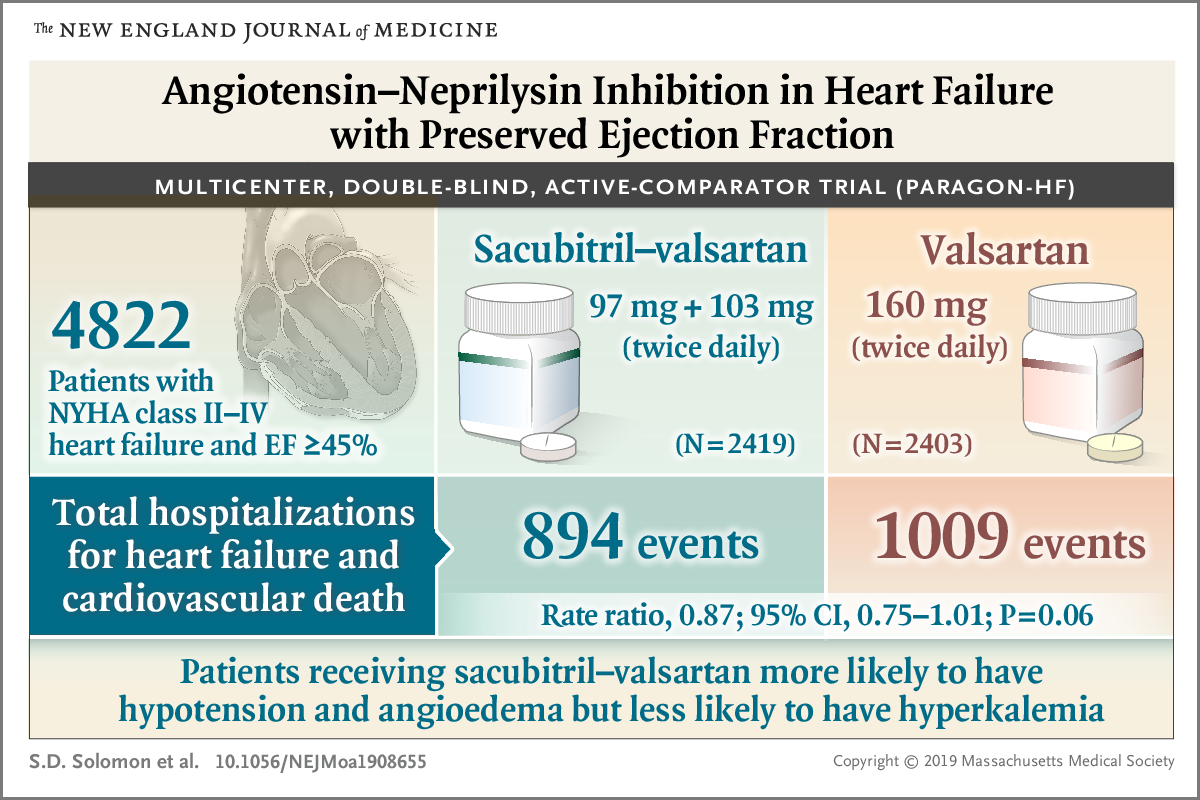
The number of patients being diagnosed with heart failure (HF) is increasing worldwide, and thus we need to know which medications to avoid or be cautious with prescribing that may cause or exacerbate this medical condition. So, we decided to talk about these medications, how they cause these adverse events in these patients, and their mechanism of action.
How these medications cause adverse events in HF patients?
Overall, these medications might cause these adverse effects by one of the following mechanisms: 1) causing direct myocardial toxicity; 2) by negative inotropic effect; 3) chronotropic effects; 4) by exacerbating hypertension; 5) by delivering a high sodium load; or 6) by drug-drug interactions that limit the beneficial effects of HF medications.
Here, we will talk briefly about the common medication classes that should be avoided in heart failure and their mechanism of causing these adverse events.
1. Non-dihydro Calcium Channel Blockers (CCB):
Including (diltiazem and verapamil) è have a negative inotropic effect, thus might increase adverse outcomes [1].
2. Nonsteroidal Anti-Inflammatory Drugs (NSAID) : Diclofenac , indomethacin , ketorolac ..etc AND COX-2 selective inhibitors (Celecoxib) :
Commonly Dispensed as over the counter drugs or as anti-inflammatory prescribed drugs è these medications are associated with increased risk of HF exacerbation, causing decline in renal function, and peripheral vasoconstriction; as such they can attenuate the efficacy and enhance the toxicity of diuretics and angiotensin converting enzyme inhibitors [2].
US Boxed Warning regarding Serious cardiovascular risk: (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction (MI), and stroke, which can be fatal. This risk may occur early in the treatment and may increase with duration of use. Celecoxib is contraindicated in the setting of coronary artery bypass graft (CABG) surgery [2].
3. Some Oral Hypoglycemic Agents:
Thiazolidinediones such as Pioglitazone è are associated with fluid retention
US Boxed Warning: Thiazolidinediones may cause or exacerbate heart failure è closely monitor for signs and symptoms of HF particularly after initiation or dose increases. If HF develops, treat and consider dose reduction or discontinuation of pioglitazone. Initiation of therapy is contraindicated in patients with NYHA class III or IV HF [3].
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors: Sitagliptin, saxagliptin, and linagliptin
In a scientific statement from American Heart Association (AHA) , saxagliptin has been determined to be an agent that may exacerbate underlying myocardial dysfunction , 2016 . The ADA recommends avoiding the use of saxagliptin in patients with HF, 2020) [4].
Bies ( Metformin ) is associated withguanid lactic acidosis, which can be fatal in patients with CHF
US Boxed Warning regarding Lactic acidosis: Risk factors include renal impairment, ≥65 years and hypoxic states, e.g: acute congestive heart failure. Metformin may be used in patients with stable heart failure, ADA 2020 [5].
4. Tumor Necrosis Factor alpha inhibitors (Anti-TNF-alpha):
Including infliximab, etanercept, and adalimumab.
Use with caution in patients with mild HF (NYHA class I, II) or decreased left ventricular function. Infliximab doses >5 mg/kg are contraindicated with moderate to severe HF (NYHA class III/IV). In a scientific statement from AHA, TNF blockers have been determined to cause either direct myocardial toxicity or exacerbate underlying myocardial dysfunction, 2016 [6,7].
5. Antiarrhythmic medications:
Class I: Flecainide, disopyramide [8]
Class III: Dronedarone, Sotalol [8]
6. Anti- Cancer medications:
Anthracyclines: doxorubicin, Daunorubicin, Mitoxantrone
US Boxed Warning: Myocardial damage (including acute left ventricular failure) can occur with doxorubicin with incidences from 1% to 20% for cumulative doses from 300 mg/m2 to 500 mg/m2 when administered every 3 weeks è monitor LVEF before, during and after treatment [9]
Targeted therapy: Bevacizumab, Lapatinib, Trastuzumab
US Boxed Warning: Trastuzumab is associated reductions in left ventricular ejection fraction (LVEF) and heart failure; the incidence is highest in patients receiving trastuzumab with an anthracycline-containing chemotherapy regimen è Evaluate LVEF in all patients prior to and during treatment; discontinue for cardiomyopathy [10]
7. Cilostazol
This is a selective inhibitor of phosphodiesterase type 3, antiplatelet and vasodilatory agent used primarily in patients with intermittent claudication and peripheral arterial disease [11].
US Boxed Warning: Cilostazol is contraindicated in patients with heart failure of any severity è causing decreased survival in patients with class III to IV heart failure [11].
8. Anti-depressant drugs:
Citalopram, Tricyclic antidepressants (TCA) such as amitriptyline, Imipramine … etc.
- TCAuse with extreme caution in patients with a history of CVD or family history of sudden death, dysrhythmias, or conduction abnormalities. In a scientific statement from AHA, TCA has been determined to exacerbate underlying myocardial dysfunction,2016 è monitor EKG [12]
- Citalopram risk of dose-dependent QT prolongation ECG and torsade de pointes (TdP) . Risk factors include Structural heart disease, e.g: MI or HF [12]
9. α1 -Blockers:
Such as prazosin and doxazosin
In a scientific statement from the AHA, -Zosin has been determined to exacerbate underlying myocardial dysfunction , 2016 [13].
10. Pregabalin
Peripheral edema may occur in patients with or without a prior history of heart failure, which may result in acute decompensated heart failure. Risk factors: Pre-existing heart failure (NYHA Class III or IV) (cautious use recommended due to limited data in this patient population [14]
11. Beta-blockers (except those approved for HF treatment: Metoprolol, Bisoprolol, Carvedilol) [15]
12. Selected Intravenous and Oral Medications High in Sodium content:
- Oral meds: Alendronate effervescent tablet, Sodium polystyrene sulfonate suspension, Polyethylene glycol powder for solution, erythromycin
- Injection meds: Piperacillin/tazobactam, Metronidazole, Ticarcillin/clavulanate, azithromycin
13. Trimethoprim-sulfamethoxazole (TMP/SMX)
==> by increasing the risk of Hyperkalemia which can be life-threatening [16].
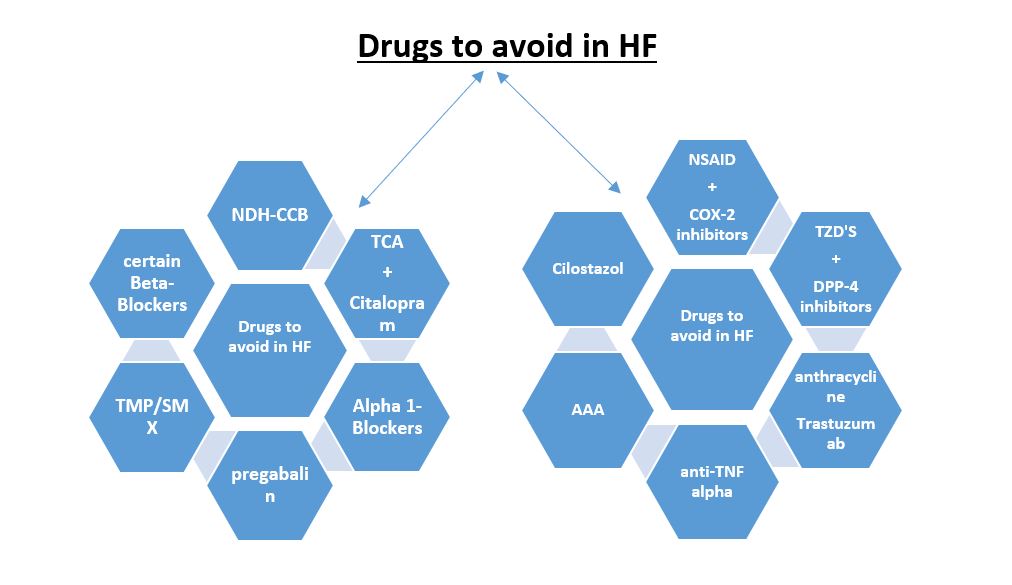
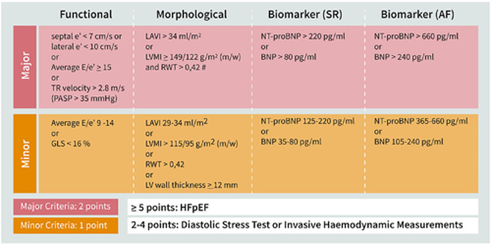
Figure 1: Summary of medications to avoid in heart failure patients.
AAA: anti-arrhythmic agents
A special thank you to my sister, Pharm.D Rawan Ya’acoub, Clinical pharmacist and Research assistant at Jordan university .
References:
[1] Kostis JB, Lacy CR, Cosgrove NM, Wilson AC. Association of calcium channel blocker use with increased rate of acute myocardial infarction in patients with left ventricular dysfunction. Am Heart J. 1997 May;133(5):550-7. doi: 10.1016/s0002-8703(97)70150-9. PMID: 9141377.
[2] Ungprasert P, Srivali N, Kittanamongkolchai W. Non-steroidal anti-inflammatory drugs and risk of heart failure exacerbation: A systematic review and meta-analysis. Eur J Intern Med. 2015 Nov;26(9):685-90. doi: 10.1016/j.ejim.2015.09.012. Epub 2015 Oct 1. PMID: 26427540.
[3] Singh S, Loke YK, Furberg CD. Thiazolidinediones and heart failure: a teleo-analysis. Diabetes Care. 2007 Aug;30(8):2148-53. doi: 10.2337/dc07-0141. Epub 2007 May 29. PMID: 17536074.
[4] Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL; SAVOR-TIMI 53 Steering Committee and Investigators*. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014 Oct 28;130(18):1579-88. doi: 10.1161/CIRCULATIONAHA.114.010389. Epub 2014 Sep 4. Erratum in: Circulation. 2015 Oct 13;132(15):e198. PMID: 25189213.
[5] Kinsara AJ, Ismail YM. Metformin in heart failure patients. Indian Heart J. 2018 Jan-Feb;70(1):175-176. doi: 10.1016/j.ihj.2017.05.009. Epub 2017 May 15. PMID: 29455774; PMCID: PMC5902828.
[6] Behnam SM, Behnam SE, Koo JY. TNF-alpha inhibitors and congestive heart failure. Skinmed. 2005 Nov-Dec;4(6):363-8. doi: 10.1111/j.1540-9740.2005.04502.x. PMID: 16276152.
[7] Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003 Jul 1;107(25):3133-40. doi: 10.1161/01.CIR.0000077913.60364.D2. Epub 2003 Jun 9. PMID: 12796126.
[8] Køber L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, Amlie J, Carlsen J; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008 Jun 19;358(25):2678-87. doi: 10.1056/NEJMoa0800456. Erratum in: N Engl J Med. 2010 Sep 30;363(14):1384. PMID: 18565860.
[9] Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019 Jun 1;307:41-48. doi: 10.1016/j.toxlet.2019.02.013. Epub 2019 Feb 25. PMID: 30817977.
[10] Nemeth BT, Varga ZV, Wu WJ, Pacher P. Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br J Pharmacol. 2017 Nov;174(21):3727-3748. doi: 10.1111/bph.13643. Epub 2016 Nov 25. PMID: 27714776; PMCID: PMC5647179.
[11] Wu CK, Lin JW, Wu LC, Chang CH. Risk of Heart Failure Hospitalization Associated With Cilostazol in Diabetes: A Nationwide Case-Crossover Study. Front Pharmacol. 2019 Jan 7;9:1467. doi: 10.3389/fphar.2018.01467. PMID: 30666197; PMCID: PMC6330376
[12] Teply RM, Packard KA, White ND, Hilleman DE, DiNicolantonio JJ. Treatment of Depression in Patients with Concomitant Cardiac Disease. Prog Cardiovasc Dis. 2016 Mar-Apr;58(5):514-28. doi: 10.1016/j.pcad.2015.11.003. Epub 2015 Nov 10. PMID: 26562328.
[13] Hundemer GL, Knoll GA, Petrcich W, Hiremath S, Ruzicka M, Burns KD, Edwards C, Bugeja A, Rhodes E, Sood MM. Kidney, Cardiac, and Safety Outcomes Associated With α-Blockers in Patients With CKD: A Population-Based Cohort Study. Am J Kidney Dis. 2021 Feb;77(2):178-189.e1. doi: 10.1053/j.ajkd.2020.07.018. Epub 2020 Sep 11. PMID: 32920153.
[14] Lund M, Poulsen G, Pasternak B, Worm Andersson N, Melbye M, Svanström H. Use of Pregabalin and Worsening Heart Failure: A Nationwide Cohort Study. Drug Saf. 2020 Oct;43(10):1035-1044. doi: 10.1007/s40264-020-00969-6. PMID: 32651945
[15] Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand J, Packer M, Coats AJS, Manzano L, Böhm M, van Veldhuisen DJ, Andersson B, Wedel H, von Lueder TG, Rigby AS, Hjalmarson Å, Kjekshus J, Cleland JGF; Beta-Blockers in Heart Failure Collaborative Group. Heart Rate and Rhythm and the Benefit of Beta-Blockers in Patients With Heart Failure. J Am Coll Cardiol. 2017 Jun 20;69(24):2885-2896. doi: 10.1016/j.jacc.2017.04.001. Epub 2017 Apr 30. PMID: 28467883.
[16] Michel A, Martín-Pérez M, Ruigómez A, García Rodríguez LA. Risk factors for hyperkalaemia in a cohort of patients with newly diagnosed heart failure: a nested case-control study in UK general practice. Eur J Heart Fail. 2015 Feb;17(2):205-13. doi: 10.1002/ejhf.226. Epub 2015 Jan 10. PMID: 25581138.
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”
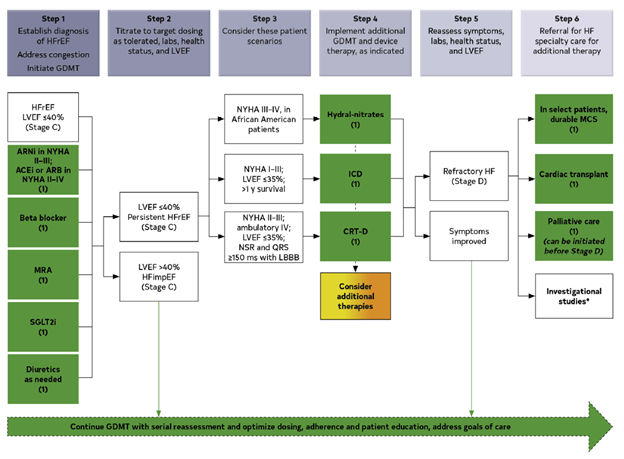
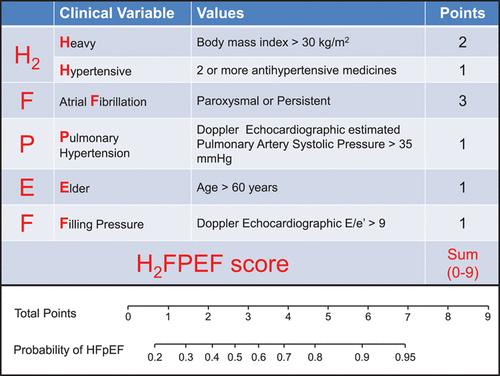

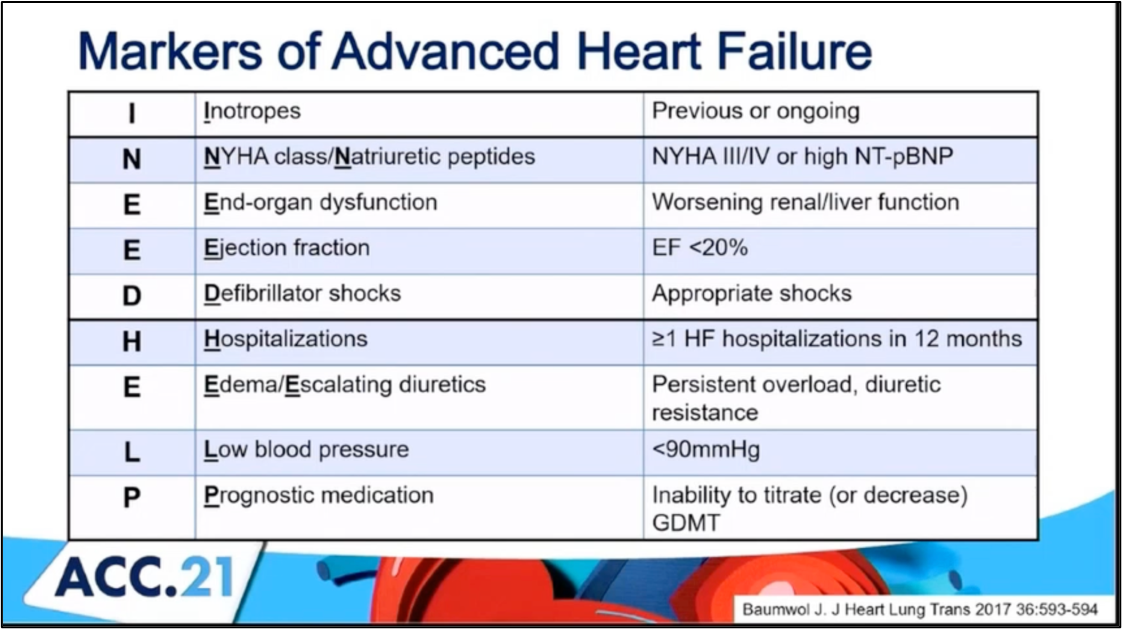
 #ACC21 came and went, bringing the usual flurry of practice-changing clinical trials, new scientific theories and inquiries, and a wealth of creative ideas showcased through poster presentations. While the virtual format is quite the departure from the in-person atmosphere, it allows flexibility in viewing sessions on-demand and allows individuals that may have an otherwise challenging time traveling to join the discussion. Aside from the trials and presentations that got the most headlines, I wanted to highlight a talk within the advanced heart failure space that expanded on a challenging clinical scenario we encounter routinely. This blog contains screenshots that are directly from the talk Moving Beyond NYHA Class: Risk Stratification and Prognosis in Advanced Heart Failure (within Session 603 The Advanced Heart Failure Therapies of LVAD and Transplant: Who, What, When, Where, Why, and How?) by Dr. Garrick Stewart from Brigham and Women’s Hospital.
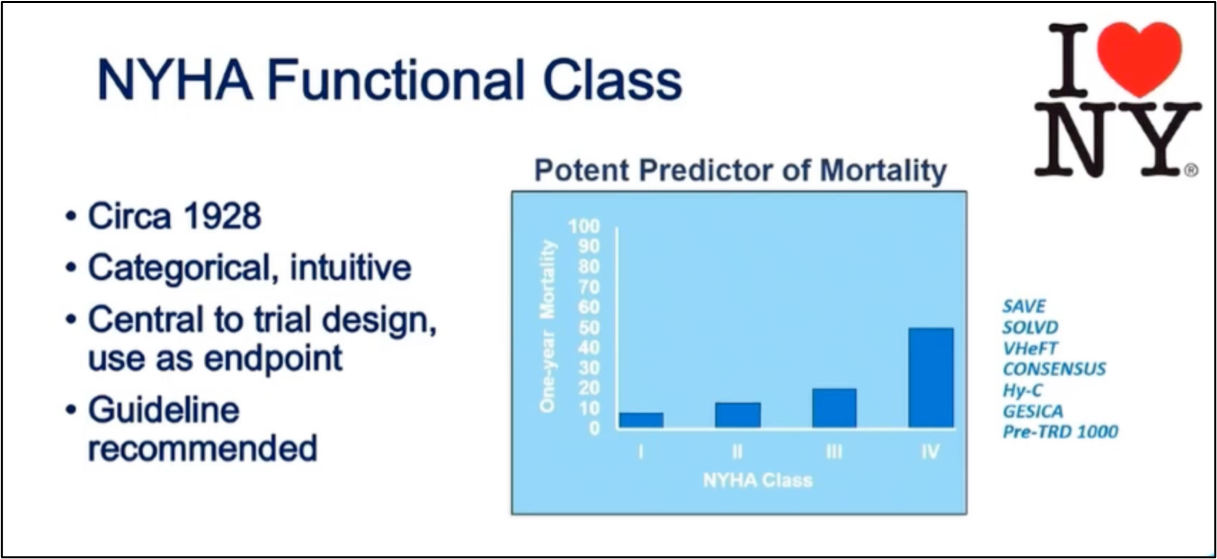
#ACC21 came and went, bringing the usual flurry of practice-changing clinical trials, new scientific theories and inquiries, and a wealth of creative ideas showcased through poster presentations. While the virtual format is quite the departure from the in-person atmosphere, it allows flexibility in viewing sessions on-demand and allows individuals that may have an otherwise challenging time traveling to join the discussion. Aside from the trials and presentations that got the most headlines, I wanted to highlight a talk within the advanced heart failure space that expanded on a challenging clinical scenario we encounter routinely. This blog contains screenshots that are directly from the talk Moving Beyond NYHA Class: Risk Stratification and Prognosis in Advanced Heart Failure (within Session 603 The Advanced Heart Failure Therapies of LVAD and Transplant: Who, What, When, Where, Why, and How?) by Dr. Garrick Stewart from Brigham and Women’s Hospital.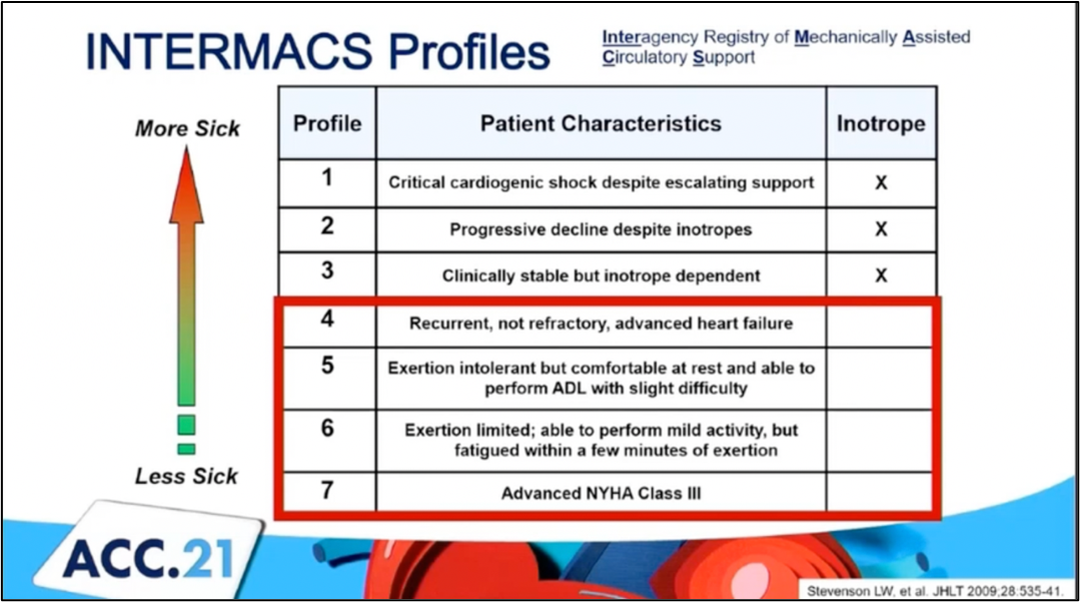 Dr. Stewart starts with an overview of how we think about and classifies patients who have heart failure, starting with the history of the New York Heart Association Class grading schema. While it is simple to use and universally known, its limited in its ability to discriminate how sick those with heart failure truly are. Specifically, it cannot tell you who is at the highest risk for morbidity and mortality. To try and address those specifically with advanced heart failure, the INTERMACS Profiles were created. He outlines in his talk how these schemes are related
Dr. Stewart starts with an overview of how we think about and classifies patients who have heart failure, starting with the history of the New York Heart Association Class grading schema. While it is simple to use and universally known, its limited in its ability to discriminate how sick those with heart failure truly are. Specifically, it cannot tell you who is at the highest risk for morbidity and mortality. To try and address those specifically with advanced heart failure, the INTERMACS Profiles were created. He outlines in his talk how these schemes are related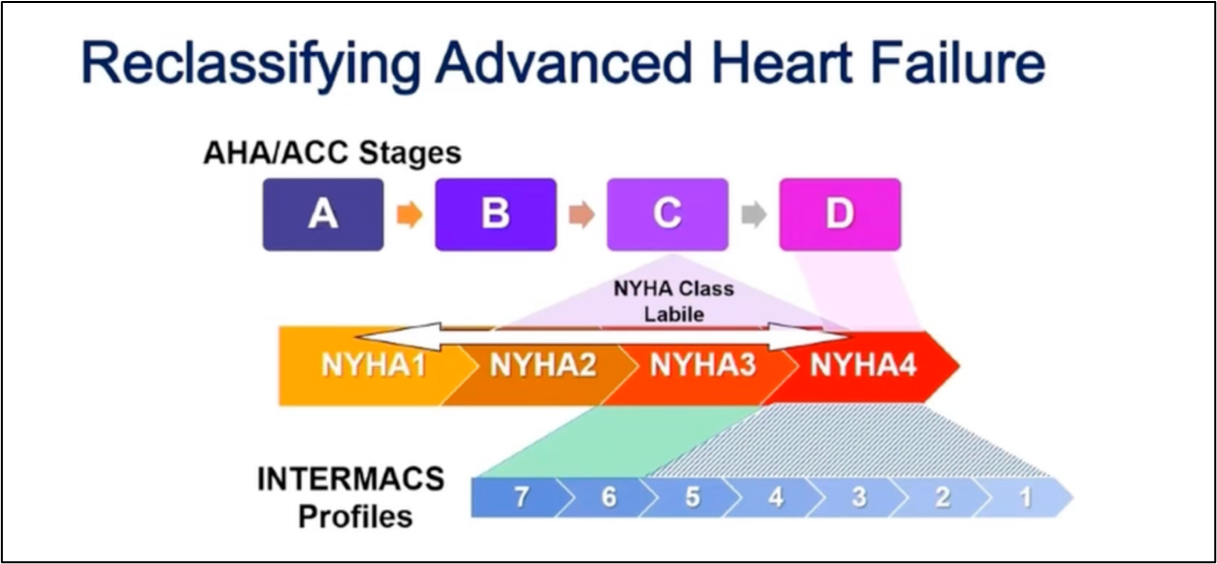
 Reference:
Reference: