Lay of the Land
In 2008, after years of being the third-leading cause of death in the United States, stroke dropped to fourth. In part, this reflected the results of a commitment made by the American Heart Association/American Stroke Association (AHA/ASA) more than a decade prior to reduce stroke, coronary heart disease, and cardiovascular risk by 25% by the year 2010 (a goal met a year early in 2009). The reason for the success, although multifactorial, can largely be attributed to improved prevention and improved care within the first hours of acute strokes.1 As early as 2000, the benefits of time-dependent administration of intravenous tissue plasminogen activator (tPA) in patients with acute ischemic stroke were well supported (Figure 1).2
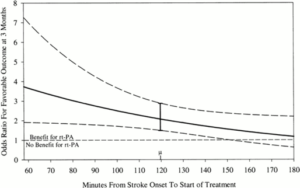
Figure 1. Graph of model estimating OR for favorable outcome at 3 months in recombinant tissue-type plasminogen activator (rt-PA) treated patients compared to placebo treated patients by time from stroke onset to treatment (onset-to-treatment time [OTT]) with 95% confidence intervals, adjusting for the baseline NIH Stroke Scale. OR > 1 indicates greater odds that rt-PA treated patients will have a favorable outcome at 3 months compared to the placebo treated patients. Range of OTT was 58 to 180 minutes with mean (μ) of 119.7 minutes.2
Guidelines began recommending a door-to-needle time for tPA administration of 60 minutes or less, however, studies found that less than 30% of US patients were treated within this time window. The Target: Stroke initiative was launched in 2010 to assist hospitals in providing timely tPA. As a result, the proportion of tPA administered within 60 minutes increased from 26.5% during the preintervention period to 41.3% after implementation. Despite national initiatives, shorter door-to-needle times have not been as quickly adopted as door-to-balloon times for percutaneous coronary intervention in acute coronary syndromes (Figure 2).4 Part of the problem is a lack of robust mortality outcomes data to support trends observed in the (only) two randomized trials conducted to assess long term outcomes with tPA in acute ischemic stroke; neither of which was powered to probe for mortality effects.
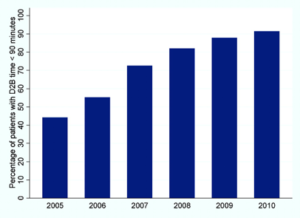
Figure 2. Trend in percentage of patients with door-to-balloon (D2B) time <90 minutes over 6 years.4
This brings us to the study published earlier this week in JAMA Man S et al. (corresponding author Fonarow GC) titled “Association Between Thrombolytic Door-to-Needle Time and 1-Year Mortality and Readmission in Patients With Acute Ischemic Stroke.” This nationwide study of US patients treated with intravenous tPA for acute ischemic stroke demonstrated that shorter door-to-needle times were significantly associated with better long-term outcomes, including lower 1-year all-cause mortality, 1-year all-cause readmission, and the composite of all-cause mortality or readmission at 1 year.5
Study Design
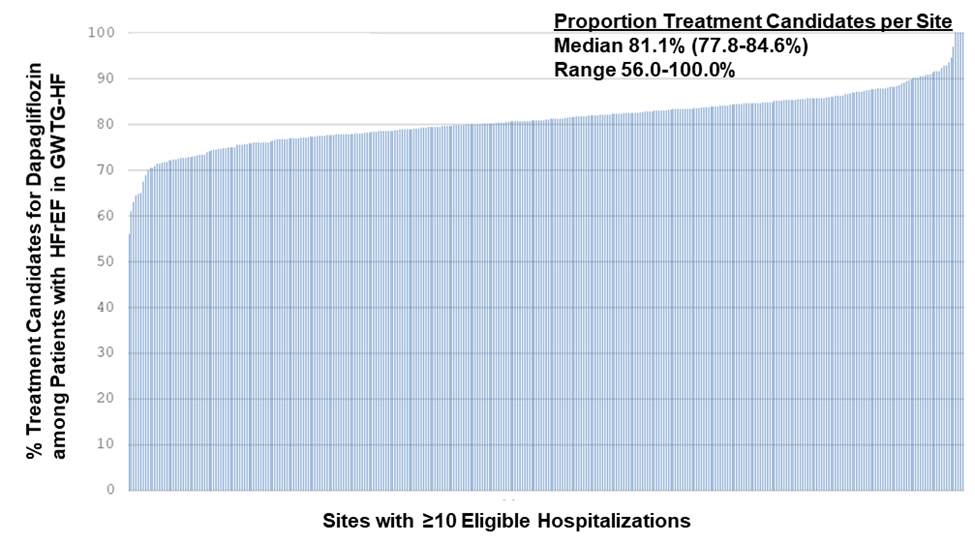
This US cohort included Medicare beneficiaries aged 65 years or older who were treated with intravenous tPA for acute ischemic stroke at Get With The Guidelines (GWTG)–Stroke participating hospitals between January 1, 2006, and December 31, 2016, with 1-year follow-up through December 31, 2017. Patient clinical data were obtained from the GWTG-Stroke database. Study entry criteria required patients to (1) have been aged 65 years or older; (2) have a discharge diagnosis of acute ischemic stroke; (3) have been treated with intravenous tPA within 4.5 hours of the time they were last known to be well; (4) have had a documented door-to-needle time; (5) not have been treated with a concomitant therapy with intra-arterial reperfusion techniques; (6) have had the admission be the first for stroke during the study period; and (7) not have been transferred to another acute care hospital, left against medical advice, or without a documented site of discharge disposition.5 Overall, 61426 participants met the inclusion criteria for the study.
The prespecified primary outcomes included 1-year all-cause mortality, 1-year all-cause readmission, and the composite of all-cause mortality or readmission at 1 year. One-year cardiovascular readmission was a prespecified secondary outcome and was defined as a readmission with a primary discharge diagnosis of hypertension, coronary artery disease, myocardial infarction, heart failure, abdominal or aortic aneurysm, valvular disease, and cardiac arrhythmia. Recurrent stroke readmission, a post hoc secondary outcome, was defined as a readmission for transient ischemic attack, ischemic and hemorrhagic stroke, carotid endarterectomy or stenting, but not for direct complications of index stroke.5
Door-to-needle time was first analyzed using the prespecified times of within 45 minutes and within 60 minutes versus longer than those targets, in line with prior studies on this topic. The authors also ingeniously also evaluated time as a continuous variable, as a categorical variable in 15-minute increments using within 30 minutes as the reference group, and in 45-minute and 60-minute increments. Cox proportional hazards models were used to examine the associations of door-to-needle timeliness and each 1-year outcome with robust variance estimation to ac- count for the clustering of patients within hospitals.5 On hours were defined as 7:00 AM to 6:00 PM on any weekday. Off hours were defined as any other time, including evenings, nights, weekends, and national holidays. The authors did this because prior studies using this prespecified time cutoff have shown that presenting during off hours was associated with inferior quality of care, inferior intravenous thrombolytic treatment, and in-hospital mortality.5
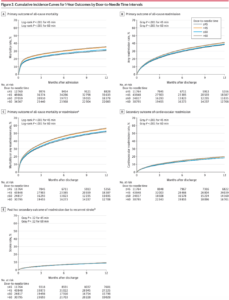
Results
Among the 61426 Medicare beneficiaries treated with intravenous tPA within 4.5 hours of the time they were last known to be well at the 1651 GWTG-Stroke participating hospitals, the median age was 80 years, 43.5% were male, 82.0% were non-Hispanic white, 8.7% were non-Hispanic black, 4.0% were Hispanic, and 5.3% were of other race/ethnicity. More patients that arrived during off hours were treated within longer door-to-needle times (40.7% for ≤30 minutes, 45.6% for 31-45 minutes, 50.6% for 46-60 minutes, 53.5% for 61-75 minutes, and 56.3% for >75 minutes; P < .001). Despite having longer onset-to-arrival times, some patients had shorter onset-to-needle and door-to-needle times.5
Most patients were treated at teaching hospitals (77.7%) and primary stroke centers (73.2%); 3% were treated at rural hospitals. More patients who were treated at teaching hospitals, but not at primary stroke centers, were treated within shorter door-to-needle times. The median door-to-needle time was 65 minutes, with 5.6% of patients treated with tPA within 30 minutes of hospital arrival, 20.8% within 45 minutes, and 44.1% within 60 minutes.5
Patients who received tPA after 45 minutes of hospital arrival had worse long-term outcomes than those treated within 45 minutes of hospital arrival, including significantly higher all-cause mortality (35.0% vs 30.8%, respectively; adjusted hazard ratio [HR], 1.13 [95% CI, 1.09- 1.18]), higher all-cause readmission (40.8% vs 38.4%; ad- justed HR, 1.08 [95% CI, 1.05-1.12]), higher all-cause mortality or readmission (56.0% vs 52.1%; adjusted HR, 1.09 [95% CI, 1.06-1.12]), and higher cardiovascular readmission (secondary outcome) (19.8% vs 18.4%; adjusted HR, 1.05 [95% CI, 1.00- 1.10]), but not significantly higher recurrent stroke readmission (a post hoc secondary outcome) (9.3% vs 8.8%; adjusted HR, 1.05 [95% CI, 0.98-1.12]).
Patients who received tPA after 60 minutes of hospital arrival vs within 60 minutes of hospital arrival had significantly higher adjusted all-cause mortality (35.8% vs 32.1%, respectively; adjusted HR, 1.11 [95% CI, 1.07-1.14]), higher all-cause readmission (41.3% vs 39.1%; adjusted HR, 1.07 [95% CI, 1.04-1.10]), higher all-cause mortality or readmission (56.8% vs 53.1%; adjusted HR, 1.08 [95% CI, 1.05-1.10]), and higher cardiovascular readmission (secondary outcome) (20.2% vs 18.6%; adjusted HR, 1.06 [95% CI, 1.01-1.10]), but not significantly higher recurrent stroke readmission (a post hoc secondary outcome) (9.3% vs 8.9%; adjusted HR, 1.03 [95% CI, 0.97-1.09]).
The absolute differences in outcomes increased with longer door-to-needle times. The cumulative incidence curves showed that approximately 42% of the deaths or readmissions occurred within 30 days.
Every 15-minute increase in door-to-needle times was significantly associated with higher all-cause mortality (adjusted HR, 1.04 [95% CI, 1.02-1.05] for door-to-needle time within 90 minutes of arrival. However, this association did not persist beyond 90 minutes of hospital arrival. Every 15-minute increase in door-to-needle times was significantly associated with higher all-cause readmission (adjusted HR, 1.02 [95% CI, 1.01- 1.03]) and higher all-cause mortality or readmission (adjusted HR, 1.02 [95% CI, 1.01-1.03]). Every 15-minute increase in door-to-needle times after 60 minutes of hospital arrival was significantly associated with higher cardiovascular readmission (secondary outcome) (adjusted HR, 1.02 [95% CI, 1.01- 1.04]) and higher stroke readmission (a post hoc secondary out- come) (adjusted HR, 1.02 [95% CI, 1.00-1.04]); however, these associations were not statistically significant for the door-to-needle times within 60 minutes of hospital arrival.
My Take
I would first like to commend the authors on this undertaking. The fact that early door-to-balloon time is still questionable seems contrary to our understanding of ischemic events and time to cell necrosis. This high-quality study further supports the notion that “time is muscle,” as seen in other ischemic events such as acute myocardial infarction and acute limb ischemia. However, the limitations of the study affects its generalizability and application to real world scenarios. The patients in this study are all over the age of 65, largely non-Hispanic whites, all with recorded times of last seen normal and mostly treated in academic centers with stroke units. Nonetheless, the authors have certainly progressed the field of stroke treatment, if even incrementally, in the right direction.
References:
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
- Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000;55(11):1649-1655.
- Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311(16):1632- 1640. doi:10.1001/jama.2014.3203
- Krumholz HM, et al. Improvements in door-to-balloon time in the United States, 2005-2010. Circulation 2011;124:1038-45.
- Man S, Xian Y, Holmes DN, et al. Association Between Thrombolytic Door-to-Needle Time and 1-Year Mortality and Readmission in Patients With Acute Ischemic Stroke. JAMA. 2020;323(21):1-15. doi:10.1001/jama.2020.5697
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”