I am pleased to have the opportunity to summarize an important recent paper on the use of sodium-glucose co-transporter 2 (SGLT2) inhibitors by Drs. Muthiah Vaduganathan, Gregg Fonarow, and colleagues in JAMA Cardiology,1 that was published simultaneously with AHA20.
Background:
SGLT2 inhibitors are a class of medications that were initially developed for management of diabetes but were serendipitously found to be effective in treating individuals with heart failure. In May 2020, dapagliflozin became the first SGLT2 inhibitor approved by the US Food and Drug Administration (FDA) for use in patients with heart failure with reduced ejection fraction (HFrEF) after the pivotal Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, which showed that dapagliflozin reduced heart failure events and mortality.2 In the EMPEROR-Reduced (EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction) trial, use of another SGLT2 inhibitor, empagliflozin, was also found to reduce risk of cardiovascular death and heart failure hospitalizations.3
Major Question Addressed in the Paper: What proportion of contemporary patients with HFrEF in the US are potentially eligible for initiation of dapagliflozin based on the FDA label?
Approach: The investigators studied patients with HFrEF (EF≤40%) who were in the AHA Get With The Guidelines-Heart Failure (GWTG-HF) registry. They assessed patients admitted between January 2014 to September 2019 at 529 sites (started with 586,580 patients). Patients were excluded if they had any of the following based on the FDA label for dapagliflozin: estimated glomerular filtration rate [eGFR]<30 mL/min/1.73 m2 at discharge, dialysis (either history of chronic dialysis or required dialysis during hospitalization), and/or type 1 diabetes. After excluding patients who met the aforementioned criteria and those who had missing discharge eGFR or vital signs, the primary study cohort consisted of 154,714 patients at 406 sites.
Major Results:
- Of the 154,714 patients studied in the GWTG-HF registry, 125,497 (81.1%) were candidates for initiation of dapagliflozin based on the FDA label.
- When only looking at sites with ≥10 hospitalizations (355 sites that enrolled 154,522 patients), the median proportion of dapagliflozin candidates was still 81.1% (25th-75th percentiles 77.8-84.6%).
- A higher proportion of patients without type 2 diabetes than with type 2 diabetes were candidates for dapagliflozin (85.5% vs. 75.6%).
- The most frequent reason for not meeting the FDA label was eGFR<30 mL/min/1.73 m2, which was met more frequently in patients with a history of or new diagnosis of diabetes than those without diabetes (23.9% vs. 14.3%).
- There was lower use of evidence-based heart failure therapies in the GWTG-HF patients compared to patients in the DAPA-HF trial.
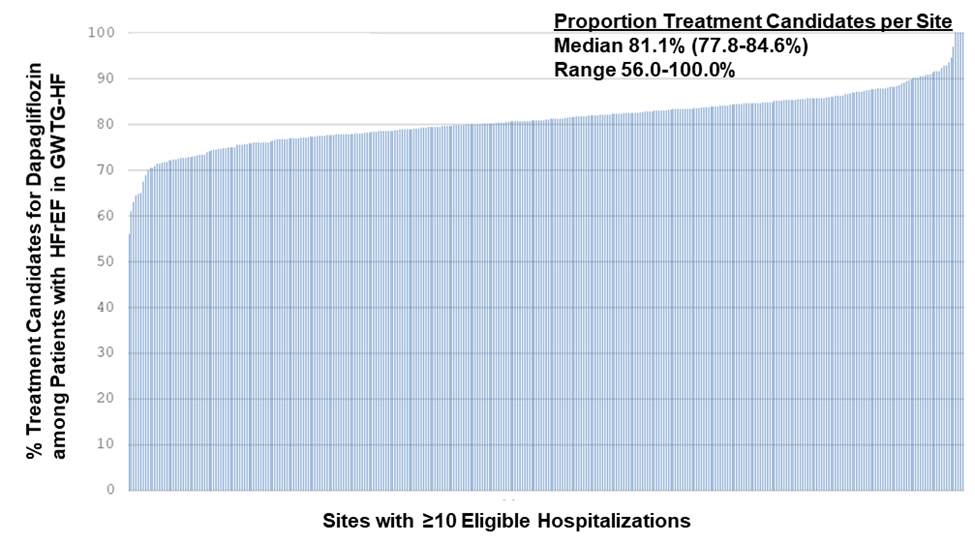
Histogram from Vaduganathan et al. evaluating the proportion of patients meeting the dapagliflozin FDA label criteria from hospitals with at least 10 eligible HFrEF hospitalizations.
Major Study Limitations: Since the GWTG-HF data are de-identified, only unique hospitalization episodes were presented so some patients may be represented more than once in this study. Glycated hemoglobin levels were not measured in a protocolized way, thus type 2 diabetes could be underdiagnosed in this study. Data regarding post-discharge labs and the use of therapies were not available.
Key Take Home Message: This study using a large AHA registry (GWTG-HF) strikingly found that 4 out of 5 adults with HFrEF (regardless of whether the patient has type 2 diabetes) may be eligible for initiation of dapagliflozin, supporting the broad applicability of this therapy in US clinical practice.
For further learning, there are several great OnDemand sessions from AHA20 on SGLT2 inhibitors.
AHA20 OnDemand Sessions on SGLT-2 inhibitors:
- New Glucose-Lowering Agents with CV Benefits: Working… But How?
- SGLT2i for Non-Diabetic Indications: Updates from Mega-Trials and Mechanistic Insights
- Novel Anti-Diabetic Agents: A Tidal Wave of Change in the Cardiovascular Care of Patients with CKD
- The Heart, the Kidney, and SGLT2 Inhibition: For Clinical Trials to Patient Care
Potential Future Research Directions:
- Determine the mechanisms leading to the efficacy of SGLT2 inhibitors in HFrEF.
- Investigate the renal effects of SGLT2 inhibitors and whether SGLT2 inhibitors can be safely used in patients with more severe chronic kidney disease.
- DAPA-CKD4 (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease), which included patients with eGFR as low as 25 mL/min/1.73 m2, showed that dapagliflozin reduced risk of sustained eGFR decline of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes regardless of the presence or absence of type 2 diabetes.
- EMPEROR-Reduced included HFrEF patients with eGFR as low as 20 mL/min/1.73 m2.
- Evaluate whether SGLT2 inhibitors are beneficial in patients with heart failure with preserved ejection fraction (HFpEF). Current ongoing/future clinical trials with HFpEF patients include DELIVER (NCT03619213), EMPEROR-Preserved (NCT03057951), EMPA-HEART 2 (NCT04461041), PRESERVED-HF (NCT03030235), and EMBRACE-HF (NCT03030222).
- Of note, EMPERIAL-Preserved (NCT03448406) did not show a significant difference in its primary endpoint, exercise capacity, in patients with HFpEF who took empagliflozin (data from the following press release: https://www.boehringer-ingelheim.us/press-release/full-results-emperial-exercise-ability-trials-presented).
- Assess the effects of simultaneous use of SGLT2 inhibitors and another class of diabetic medications that have shown beneficial cardiovascular disease (CVD) effects, glucagon-like peptide-1 receptor agonists (GLP-1RA) and determine which of these two classes of medications should be prioritized in drug-naïve patients with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD).
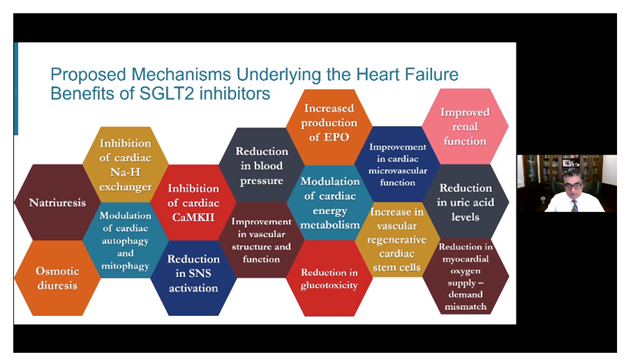
Potential mechanisms underlying the beneficial effects of SGLT2 inhibitors. Figure from Dr. Subodh Verma’s talk entitled “SGLT2 inhibitors: Why do they work” in the “New Glucose-Lowering Agents with CV Benefits: Working… But How?” session at AHA20.
References
- Vaduganathan M, Greene SJ, Zhang S, Grau-Sepulveda M, DeVore AD, Butler J, Heidenreich PA, Huang JC, Kittleson MM, Joynt Maddox KE, McDermott JJ, Owens AT, Peterson PN, Solomon SD, Vardeny O, Yancy CW, Fonarow GC. Applicability of us food and drug administration labeling for dapagliflozin to patients with heart failure with reduced ejection fraction in us clinical practice: The get with the guidelines-heart failure (gwtg-hf) registry. JAMA Cardiol. 2020
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, Investigators D-HTCa. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, Investigators E-RT. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC, Investigators D-CTCa. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436-1446
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”