Almost one year since COVID19 was deemed a pandemic, we are nowhere close to get it under control. Although it has affected the healthcare system in innumerable ways, stroke management has been particularly impacted. Not only by the disease itself, but also by the multidisciplinary and strictly protocols for its diagnosis and treatment that have been difficult to maintain for the past year. To understand the impact of COVID19 on stroke management, Dr. Shlee S. Song, the Director of the Comprehensive Stroke Center at Cedars-Sinai shared with me her experiences, and learnings through these unprecedented times.
Dr. Shlee S. Song, Director of is a board-certified vascular neurologist who completed her clinical and research fellowship at the National Institutes of Health. She has worked on steering committees and served as PI on multiple national and international multicenter stroke clinical trials. She serves as Medical Director of Stroke Programs at Cedars Sinai, Torrance Memorial, and Marina del Rey hospital, affiliate sites of Cedars-Sinai, and has developed a telemedicine network that delivers acute stroke care, oversees stroke quality improvement, and clinical trials enrollment across network hospitals. As the previous program director for the vascular neurology fellowship program at Cedars-Sinai, she has trained many stroke neurologists that practice across the country.
MDQC: Dr. Song, what is the association between COVID19 and Stroke?
Dr. Song: So far, we know that COVID has been associated with an inflammatory state and hypercoagulable state. Patients with more severe COVID symptoms also develop cloths in both lungs and other end organs. When we had our initial surge in the spring months in 2020, we had avoided what our NY colleagues have seen, like large vessel occlusion (LVO) in young patients. However, we did see a surge since the end of November. We have had a case series of patients where they were young, without many comorbidities, but had large cloths in large vessels like the ICA and carotid.
MDQC: So, is there an association between COVID severity and stroke?
SS: Right, what I have seen so far is that patients may have one risk factor or a couple, whether it is on birth control, having hypertension, or diabetes, even if they are managing their risk factors well, a COVID infection tips the scale toward clotting. Maybe a 2 or 3 hit hypothesis, where if you have the individual risk factors you are not in an inflammatory state, you can manage them, but when the infection occurs, the other diseases set off this cascade of injury that we see.
MDQC: Has the standard of care for management for patients with stroke has changed during the pandemic?
SS: We have seen that during the pandemic, that we have to be flexible. With the demand so high for stabilization, the surge in patients to the ICU, and hospital systems being stressed, our usual stroke pathways are not available. The patients are spread out all over the hospital because the beds are hard to come by. We have to be able to train a lot of our other service line team members to be able to deliver emergency care and monitoring.
For example, sometimes, the patients cannot go to the Neuro ICU, our usual pathway. Sometimes they are going to the PACU, where the personnel might not have received that training to get the NIH stroke scale done. However, we are focusing on the things we can monitor in more severe COVID. ICU patients that require high ventilation settings have to be paralyzed, so it doesn’t make sense to do no an usual neuromonitoring ( antigravitational strength, speech, etc.), but we can do other things like checking the pupils. We have had to shift our thinking and pivot to tailor to our situation.
Right now, we are in the “stabilization mode.” We are no trying to plan a 3-6 12 follow-up because, before the pandemic, we were able to stabilize our patients quickly. However, right now, it takes longer to stabilize these patients because of the injury to their lungs. We are just trying to get patients through to the first one or two weeks and then talk about lipid-lowering and secondary prevention that can be addressed later on. Right now, we want them to survive this cascade and storm that is going on.
MDQC: Would you consider changing the mindset of strict diagnostic and treatment protocols for stroke has been the most significant challenge during the pandemic?
SS: I think there is an acknowledgment from our specialist, that are in the frontline, that we have to be flexible because we are all operating in the dark, but we are realizing collectively that we are dealing with such limited data, this is so new in terms of what we are experiencing.
Acknowledging that there is limited data allows us to focus right now on acute stabilization and realize that somethings can be done down the road. We are working on that standardized protocol to promote this mindset to streamline the process, so during night calls, there would be some guidance focused on stabilizing the patients when there is a limited team.
MDQC: Since stroke is an acute event, what has the hospital done to procure the healthcare personnel’s safety when a patient comes to the ER with an acute stroke regarding their COVID19 status?
SS: We are minimizing the amount of exposure to our team members. Since we are a small team, we want to preserve everyone’s safety. We have incorporated our telestroke robot in our emergency department (ED). Our stroke team nurses’ expertise is well-versed in maneuvering and is quick at getting their images done and answering the inclusion/exclusion criteria for thrombolytic criteria. We can see the ER with the robot’s camera. Although we agree that is this is not equivalent to see the patient at the bedside, we are aware that we oversee a system where our stroke neurologist covers multiple hospitals, not only Cedars-Sinai.
Everyone has the personal protective equipment (PPE) ready in their backpacks, our gown, N95 masks. In the setting of a stroke code, anything can happen, sometimes the patients’ airway gets compromised or has a seizure; while this happens, we can quickly gear up since we have it with us. Our pharmacies will now have 24-hour coverage as an additional help to stabilize these patients.
The rapid COVID19 test is available. We try to do it as early during the code if we suspect an LVO, so that information can be available to the IR colleagues who can be prepared. They are also assuming that many of our patients are COVID positive. However, suppose we don’t have the test results. In that case, we don’t delay the emergency recanalization procedure, if the patient is eligible, so we assume they are positive or suspected for Covid, and we gear up properly.
MDQC: What is the impact telestroke has had in managing stroke during the pandemic? And how do you think It will evolve in the years to come?
SS: Telemedicine and telestroke are here to stay. It has been around for decades. We started our program of telestroke in 2016 for covering Torrance Memorial Hospital, and the demand keeps growing. Every minute counts in the setting of a stroke code. It doesn’t make sense for someone to start driving to a hospital when we have a camera that can quickly help guide our ER or ICU colleagues.
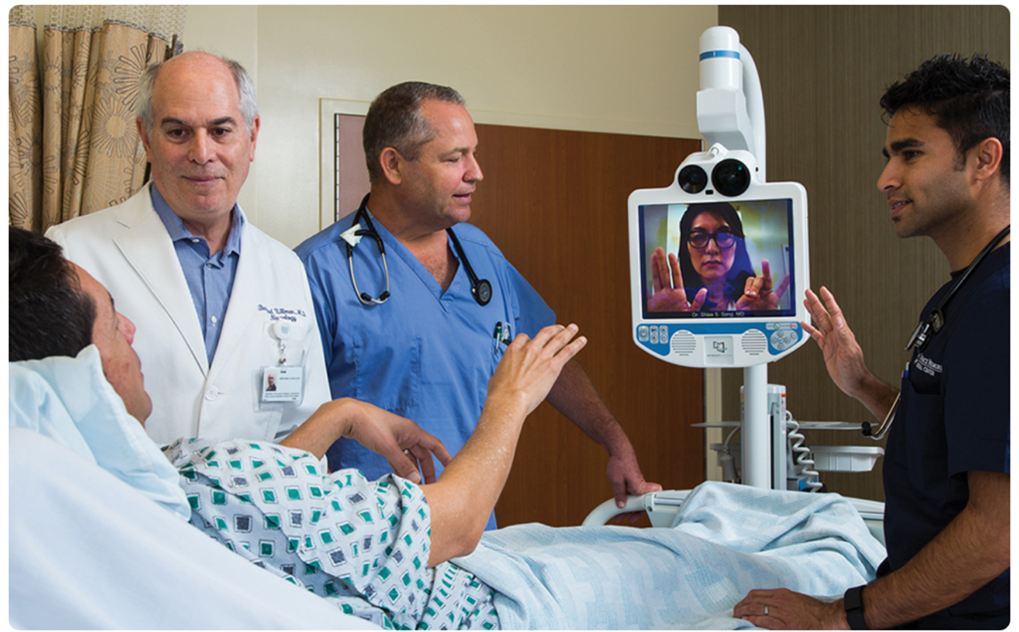
Dr. Song, pictured on the monitor, practices treating a stroke patient remotely with other members of the care team.
The technology has been around for a while. It continues to improve, like being able to see the imaging, PAC access, able to quickly document assessments, and write the recommendations that can be seen by the team members that are accepting patients in the ICU. The technology is being improved regularly, the software, and hardware, such as upgrading the camera or reducing background noise.
One thing that I have seen during the pandemic in telestroke where I would like to see some improvement in our non-speaking English patients. Especially with the pandemic and the no-visitor policy of many hospitals, out of a concern for community spread. It’s been challenging to get accurate clinical history from our patients. We rely on witnesses from the family and relatives to determine their medication, clinical history, and bleeding risk. All of that information is difficult to get, especially if we don’t get translators in the room. If Telemedicine could get paired with translator services, so they could be available during the stroke codes, I think that would help move things along from us.
MDQC: This is especially problematic given that the Latinx population has been affected disproportionally.
SS: Yes, we saw that in our data as well. We have a paper submitted right now (REFERENCE), looking at the nine-stroke comprehensive centers in Los Angeles. We saw a disproportional amount of Latinx community affected with LVO going to our colleague hospitals, and they have noticed a sharp increase in their thrombectomy volume during the pandemic.
MDQC: Why is the Latinx community disproportionally affected?
SS: We are trying to figure out what the patient profile looks like for that cohort. We don’t know exactly; however, some theories, such as having type 2 diabetes, maybe factor in the clotting cascade in patients with COVID. Additionally, the situation with multigenerational housing and the high prevalence of essential workers within this community don’t allow them to shelter at home because they still need to go to work. These factors have been considered to contribute to stroke, but there is no known causal relationship to date.
MDQC: Nonetheless, the social determinants play a massive role in the LatinX community.
SS: Yes, and we have been seen this in feedback from our patients. For many patients getting their health maintenance evaluation is hard since they have not had their medication for HTA, DM, etc. Chronic diseases are not being controlled. Some of them haven’t seen their doctors since most clinical visits have moved to Telemedicine, which is contingent on having a computer and Wi-Fi.
In a community with many living in multigenerational households, the computers and internet might be limited resources. Sometimes they only have one computer that must be shared, for example, with kids, for distance learning, and they don’t have other devices to schedule their appointments. COVID has highlighted the gaps between the patients with more resources and those lacking them.
MDQC: We assume that everyone has a computer and good internet access, and unfortunately, that is not the reality. A pillar of medicine is the hands-on training for medical students, residents, fellows. What changes have occurred to guarantee appropriate learning during the pandemic?
SS: We have taken this opportunity to push our trainees’ telemedicine skills in the neurology residency program. Before the pandemic, we had separated telestroke training only for the fellow because we wanted the residence to have that bedside experience first before going to the telemedicine platform. We quickly realize that this skill set needed to be incorporated into the curriculum.
We wrote a paper about that and published it in Neurology, with Dr. Alicia Zha from the University of Texas and colleagues from the University of Utah.1 We have incorporated Telemedicine for the residency program. Using the telemedicine robot, our residents are directing the camera and maneuvering the robot. We also have the capability called multi presence where the attending and fellow can see what the resident is doing, so we can all see what the host resident is doing, and we can easily take over if we need to. Having this tool has been helpful and flexible. It allows the trainee to develop these skillsets for this technology that is here to stay. Other things that have improved since the pandemic are reimbursement since now Medicare allows the Telemedicine encounters to be equivalent to the side delivery of care. It has been helpful to continue to implement Telemedicine in our practice.
MDQC: So, is this being implemented just for acute stroke?
SS: The residents are using Telemedicine for the clinical encounters since we realize the virtual space is safer for both our patients and the provider. We moved much clinical evaluation to the iPad or evaluated with the desktop computer. It is also good to identify the gaps with Telemedicine, such as the subtle things with weakness and coordination we might not be able to pick up, which is very hard over the camera. Our residents are finding with their experience that things like visual fields cannot be done well with the equipment that we have right now. It’s important to know where our current gaps are so that this generation helps to problem-solve these issues to create apps or more tools to develop better telecare.
MDQC: Another colossal problem regarding stroke is the increase of the delay from symptom onset to arrival to the hospital. What has been your experience at Cedars-Sinai regarding this phenomenon?
SS: On the study with the nine-stroke comprehensive centers, we have seen that. Collectively we all had a decrease in our thrombolytic treatment patients, and IV tPA numbers have gone down, mostly the mild symptomatic patients. I think many patients and their family members are fearful. They have heard the system is currently overloaded and might think that their symptoms are very mild and not worth going to the hospital or are afraid of getting exposed to the virus. We have worked together with the AHA, Stroke association, and Los Angeles County to diffuse the message to tell people that if you have an emergency, a condition like a stroke, call 911.
Understanding patients and famile’ fear, we are trying to get patients home as soon as possible. In that streamlined workup, we intend to get patients out of this hospital as soon as possible. Suppose some things can be done outside of the hospital in the outpatient setting, then that is what we would like to do to reserve the hospital setting for the severe cases.
Some of our patients with mild symptoms when they get evaluated may have resolved their symptoms. We do urgent things for these high-risk TIA patients, such as the vessel evaluation of the carotids. However, maybe the Eco can be done in an outpatient setting, so we send the patient home with the Zio pad, telemonitoring, and have a home visit in 48-72 hours. We are more flexible in the way we deliver health. Not everything has to be delivered at the hospital, understanding patients’ fear and wanting to get home as soon as possible.
MDQC: Burning out syndrome has been a pressing issue in healthcare personnel even before the pandemic, how are you doing Dr. Song? and how are you and your peers coping with the stress this pandemic has caused?
SS: In terms of how we are manning with this crisis scenario is leaning on each other more. We have weekly check-ins, we called it our “stroke team huddles”, we have always had it because we have a very stressful job. We deal with patients and family members in a moment of crisis, are life and death situation during many of those codes, and now we are seen a lot more death.
Now there are lot more patients sicker, and we are seen more distress because they can’t have at the bedside their loved ones. How we have been dealing with ourselves? is giving each other the space to share that level of stress, so it is not something they are holding on to, but a shared collective, living process.
We have noticed that everyone has their highs and lows at different times, so we take advantage of that. The person who is doing well that day, really reaching out to say, “hey, unload a little bit, let me hear what is going on,” and for the person who is having a tougher day. Another really helpful thing has been laughter and sharing when we see something very funny; it has been really helpful to get us through. Sometimes we have to say what we are going through is so ridiculous, and just calling it out has relieved the tension when you share it, and I see it in the body language. It seems to lift from a burden and seems more relaxed.
We have counselors on checking areas, we have resources from Cedars, and if I see that something is helpful, I share it. I have been very open on how I’m getting through this crisis, either with therapy, with zoom check in with girlfriends, who are also experiencing high stress levels at home and work. All those coping mechanisms help and just check in with the clinic patients.
I have been writing letters and have encouraged residents and nurses to write letters to the patients and check in on them, especially those at higher risk because they live alone. You reach out to them, and they also give back to you and often ask how we are doing to the doctors, nurses. We are taking care of each other through this; the key is that we have each other, and we have a team approach.
MDQC: As the last question Dr. Song, what lesson have you learned from ongoing this pandemic?
SS: Lots of lessons. It has helped to solidify for me that we are doing meaningful work. In our team members, we are focusing more on what’s important and letting go of what is not. Our energy stores are getting depleted faster. We are learning to let go easier, focused on important things, and getting rid of the noise that doesn’t allow us to do so. Now we are getting more efficient in our work.
Also, we have all collectively seen that we need to do better, especially for our community areas where resources are lacking. There is a lot of goodwill and recognition in the stroke community. When it comes to leadership, we have to improve healthcare disparities. We are so grateful to the essential workers working delivering packages, in groceries, and getting us through the pandemic. They are the mail carriers, cashiers, all the people that have helped society keep moving during this pandemic. We need to give back to them.
Dr. Shlee Song shared that the pandemic has highlighted the consequences of an unequal healthcare system. We must strive to address this pressing issue as vehemently as finding new interventions or drugs. That flexibility and adaptation have been paramount to get through the pandemic. However, most important of all is teamwork, to rely on each other to provide the best care to patients and take care of each other.
Reference
- Zha AM, Chung LS, Song SS, Majersik JJ, Jagolino-Cole AL. Training in Neurology: Adoption of resident teleneurology training in the wake of COVID-19: Telemedicine crash course. Neurology. 2020;95(9):404-407.
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”