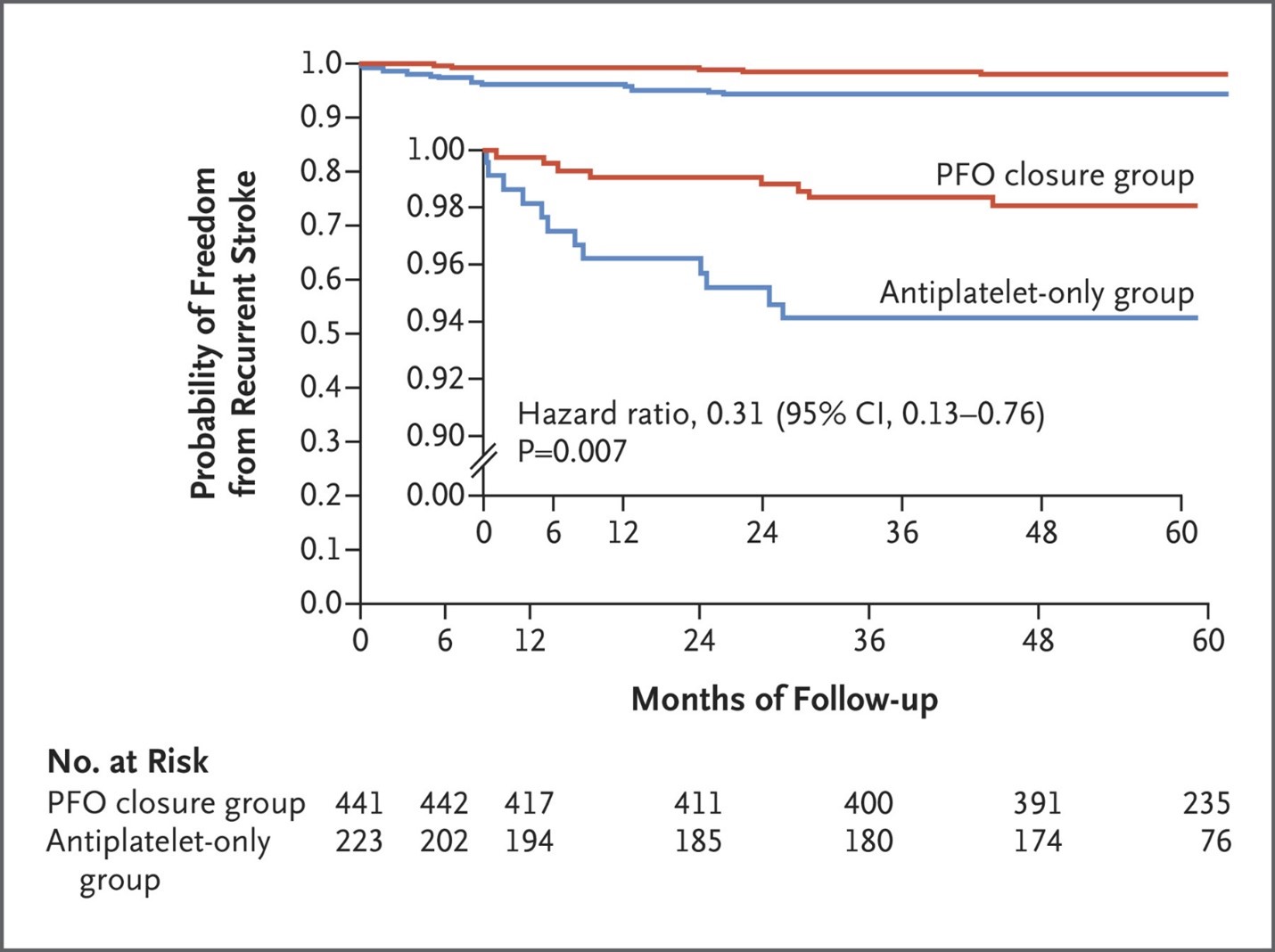
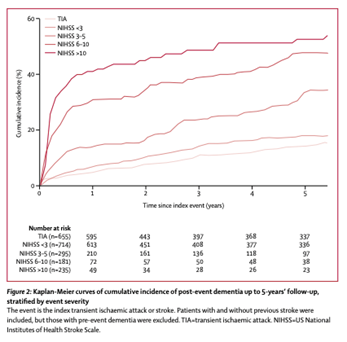
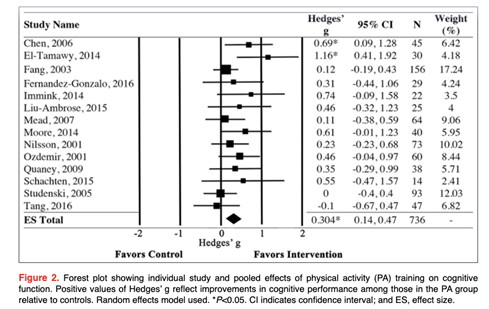
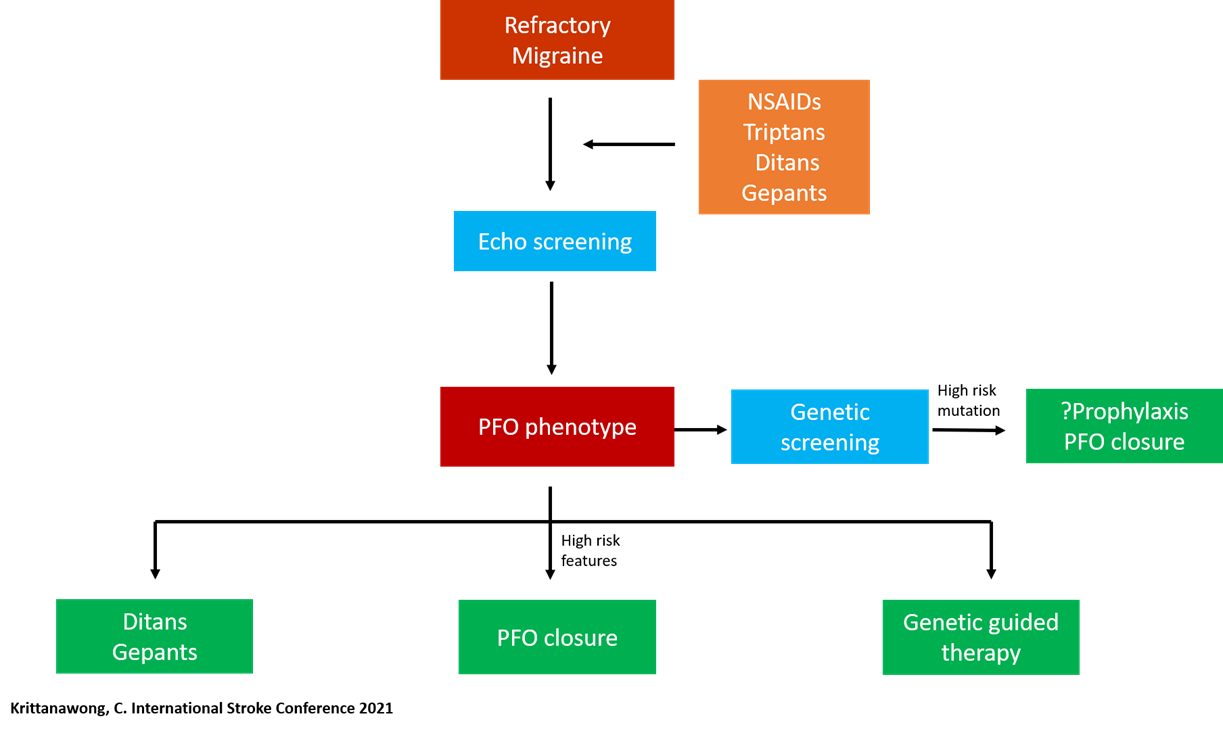
Get with the Guidelines (GWTG) – Stroke Patient Registry Use in Primary and Comprehensive Designated Stroke Centers during COVID-19 Pandemic
This year many of the professional conferences that traditionally took place live have had to change to virtual mode due to the global COVID-19 pandemic and its related social distancing rules. The International Stroke Conference and Nursing Symposium was no exception. Yet it presented an excellent opportunity for many to attend, especially those who could not have joined the conference in-person had the opportunity to participate virtually. Healthcare professionals, academicians, researchers, and supporters of stroke prevention were able to join from different places in the world, under different time zones. There were many options for participants to engage and interact in the many discussions and presentations through the online platform.
Earlier I had the opportunity to write about various topics presented during the #ISC21 (you can read them here: “Reducing Disparities through Diversity and Inclusion in Stroke Science, Clinical Trial Enrollment, and Community Engagement”; “Transformation of the GWTG – Stroke Patient Registry to into a National Representative Database of Acute Ischemic Strokes (AIS) in the U.S.”).
Today, I wanted to interview a couple of conference participants who could share with you about their experience attending this virtual conference. I also wanted them to share with you their experience with the GWTG Stroke Registry and the prevention of stroke in the midst of the COVID-19 pandemic. My guests for this post-conference interview are Ms. Jessilyn Pozo, Baptist Health South Florida System-Wide Stroke Program Manager, and Dawntray Radford, Stroke Coordinator for South Miami Hospital (You can follow them for more information here). This transcript is a lightly edited version of the interview we conducted on webcam, shortly after the 2021 International Stroke Conference.
Catherina: How was your experience at the 2021 International Stroke Conference (ISC) delivered in virtual mode?
| Dawntray: The International Stroke Conference was definitely different this year. However, I was appreciative that they (AHA) were able to extend the sessions’ timeframe so that we would be able to take a deeper dive, engage in deeper discussions opposed to the 10-15 minute sessions that we normally would have (in a live conference). I think I got a lot more information (from the presentations and discussions), especially within the different scheduled presentations. Therefore, I think there was an added bonus of extending the sessions’ timeframe.
|
 Dawntray Radford, BSN, RN Stroke Coordinator South Miami Hospital |
|
Jessilyn: This is my second time attending ISC. I went last year to Los Angeles for it. Although I do like the live version more, I liked that we were able to see lectures recorded and delivered on-demand. There were a lot of interesting topics this year, specifically hot topics with Tenecteplase1, which many hospitals are leaning towards converting its use. There were different topics like the nursing care guidelines, and reports from recent studies released. We were able to take many good notes, and we were able to pause and write down things and keep going with the lectures. I really enjoyed attending the conference, but I am excited for it to be live next year.
|
 Jessilyn Pozo, BSN, RN, SCRN BHSF System-Wide Stroke Program Manager Baptist Hospital of Miami |
Catherina: How would you describe your role in the stroke program at your organization?
Jessilyn: I oversee the stroke program for the Baptist Health system. Baptist Hospital of Miami is our comprehensive center. Dawntray Redford runs the South Miami Hospital stroke program, which is a primary stroke center, certified by the Joint Commission.2 She worked tirelessly to get it certified with no Requests for Improvements (RIFs). So kudos to her! We are working with West Kendall Baptist Hospital to become a primary stroke center. We are working to have a few of our other entities to be acute stroke ready. We have oversight of the stroke program at each individual entity and as a system to provide standardized great stroke care for all patients.
Catherina: Please tell us Ms. Radford about your role in the stroke program at South Miami Hospital.
Dawntray: We went through our first initial certification as a primary stroke center. There are a lot of moving parts in the program that we need to monitor. In addition to providing care, since we are a primary stroke center, there is an urgency of transferring stroke patients to the comprehensive center. This shows to our community and Emergency Medical Services (EMS) that we have the capabilities of readily identifying the acute stroke patients when they arrive and transferring them out at a target time of sixty minutes. Based on the feedback we received from the certification survey by the Joint Commission, it was very impressive! Because of the national times, the average goal is to push for at least 90 minutes. The literature suggests and has proven (benefits) from taking about 2 hours to 3 hours to actually have a patient transferred out to an equipped hospital. Emergency medical services (EMS) had tried to propose to bypass the primary stroke centers and go to the comprehensive one. They did not want these two-to-three-hour delays of the patient transferred because of so many logistics of trying to transfer a patient from one hospital to another system, as we had to go through that transfer process. With the streamlined process at our Institute, the Miami Neuroscience Institute, we have our own streamlined process and our dedicated transfer center. We can actually execute our transfers in sixty minutes. We worked very hard with our internal system of identifying patients before they even arrived to our institution. We are having that proactive approach of readily identifying that patient that has that large vessel occlusion. We already have a transfer center in place before the patient even arrives. This would make our numbers soar to that target timeframe for patients to get excellent stroke care. During our certification survey, we got compliments on our timeframe, less than the 90-minute-to-120-minutes timeframe, as we probably may be set back a new benchmark for the nation.
Catherina: What are the benefits of the GWTG Stroke Registry at your organizations?
Jessilyn: We are very lucky to have a data analyst team that is driven and just solely dedicated to the management of our stroke data. They are the ones who check on our stroke alert times; make these dashboards with turnaround times that they input in Get With The Guidelines. The Get With The Guidelines Stroke Registry helps us to stay on track. It keeps us on our toes, making sure that we meet the (stroke) goals. We aim to provide the care that we need to (deliver to stroke patients) based on the guidelines and the standards. This (registry data) allows for feedback on how our programs are doing.
Dawntray: The use of The Get With The Guidelines at South Miami Hospital is imperative, especially with the fact that we have different stroke units. The staff at the stroke units would like to see how they are doing as an individual unit, so they know where they need to improve individually as opposed to the hospital as a whole. Especially with the Emergency Department, their metrics would be different from the metrics of an inpatient unit. At least with the registry, I could take the different core quality measures and give the appropriate information specific to their unit. I use the registry 100% to monitor our quality measures and performance improvement measures.
Catherina: What has been your experience with stroke patients seeking stroke care in the midst of the COVID-19 pandemic?
Dawntray: We definitely have seen a decrease in the volume of care, especially with EMS and the patients that walk in. Eighty percent of our patients would arrive by their private vehicles. Many patients did not come through EMS during the pandemic. We noticed at least 50% change in our volume for at least the first two months of the COVID pandemic. We have also seen an increase in ischemic strokes with clots, with occlusive strokes in patients that were positive for COVID. They developed COVID first. The developed stroke as a secondary diagnosis.
Jessilyn: From the comprehensive center standpoint, being like the hub of the system, we have seen internal patient transfers from our sister hospitals. These patients were initially admitted for COVID care. They developed an acute ischemic stroke and were transferred over for neuro intervention. Unfortunately, these have been the trickiest patients. They were on the younger side, ended up being hypercoagulable. Our interventionalists are amazing! However, they do say it is more difficult, they find more clots. It is not just one. They seem to find several clots. These patients also tend to reocclude, even though they have had a successful thrombectomy. Therefore, I think COVID has really posed quite a challenge in stroke care for all.
Catherina: What suggestions do you have for healthcare professionals in educating patients about the prevention of stroke, especially during this COVID-19 pandemic?
Jessilyn: I think one of the biggest issues in stroke is that as high as it is, 80% of the strokes are preventable. Stroke should probably be out of the top 10 issues that are the cause of mortality in our nation or in the world. A lot of it has to do with the fact that people do not recognize the symptoms. It also has to do with getting them in here (hospital) for early treatment. We have those 24 hours for them to be a possible candidate for stroke care. A lot of them do not just even recognize the symptoms or the risk factors of stroke. They do not understand things that they just do in their daily life, that if they were to change one of these minute things, it can help them decrease their risk of stroke and relieve them from possible debilitating life symptoms.
Dawntray: (During the pandemic) we reached out to our marketing department. We have a Facebook page where we have a post on Fridays. (We posted) on recognition of the signs of early stroke: FAST: Face, Arm, Speech, Time of recognizing stroke, calling 911. We also had information on what (symptoms) to look for. We had a message built in to the post as well, stating that, “we know that you may be afraid to come in, that you want to stay at home, but you choose to be aware of, of not being afraid to seek services, to come in to the hospital where it is safe.” “We take a lot of preventative measures to protect ourselves and to the community during the pandemic”. We are just letting them know what the signs and symptoms were and not to be afraid to come in and to seek care (at the hospital). We are just giving them that comfort that it is safe to come into the hospital. Because that is what they feel… it was not safe, so they were afraid to come in (during the pandemic).
Catherina: Thank you for the opportunity to interview you and look forward to the next ICS conference. Anything that you would like to share out there with stroke coordinators, any advice or word of guidance?
Jessilyn: Just hang in there.
Dawntray: You have to be inventive. Just know that a pandemic cannot hinder you from providing the care that you provide every day. You just have to be creative, find a better way, a different way of still executing what you do on a daily basis.
I would like to thank Ms. Jessilyn Pozo and Ms. Dawntray Redford for sharing their experiences during this 2021 Virtual International Stroke conference as well as their experiences with the GWTG Stroke Registry, Primary and Comprehensive Stroke Program, and stroke prevention during the COVID-19 pandemic. For more information, you can reach them at [email protected] and [email protected]
References:
- Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase Thrombolysis for Acute Ischemic Stroke. Stroke. 2020;51(11):3440-3451. doi:10.1161/STROKEAHA.120.029749
- The Joint Commission. Primary Stroke Center Certification. (2021). Retrieved from https://www.jointcommission.org/accreditation-and-certification/certification/certifications-by-setting/hospital-certifications/stroke-certification/advanced-stroke/primary-stroke-center/
- American Heart Association. Get with the Guidelines Stroke Registry. (2021). Retrieved from https://www.heart.org/en/professional/quality-improvement/get-with-the-guidelines/get-with-the-guidelines-stroke
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”