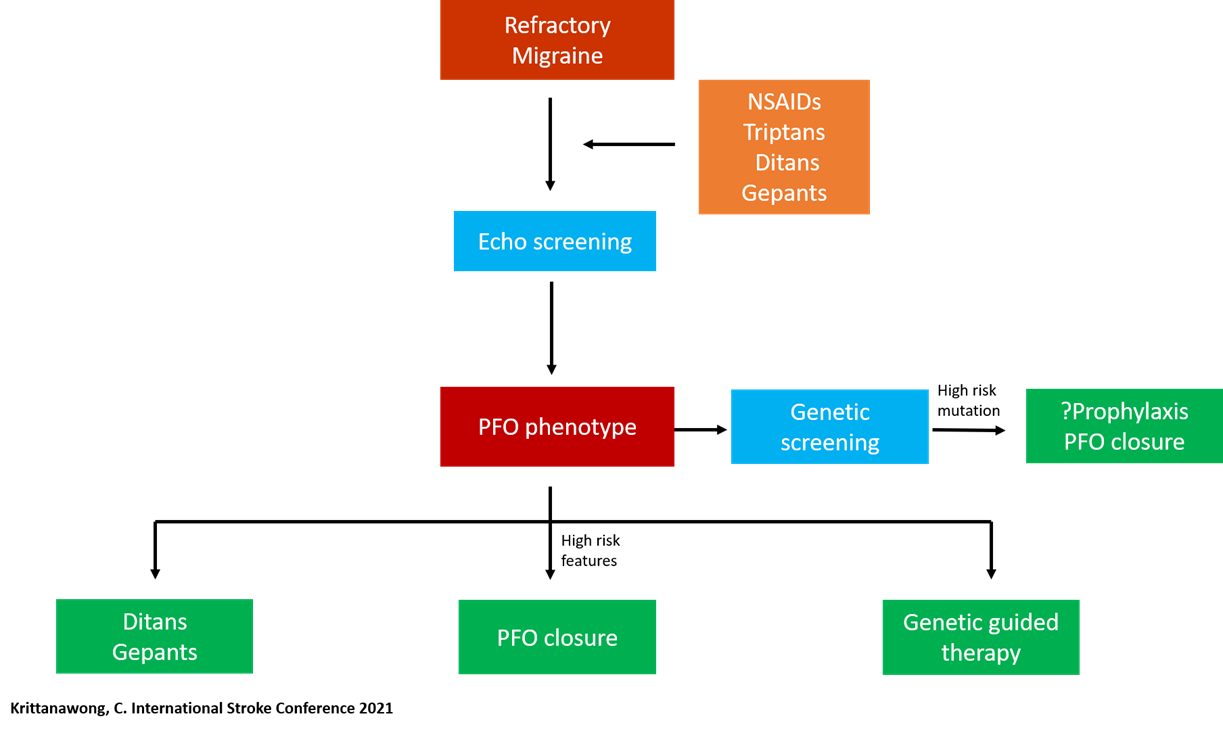
PFO Closure in PFO-related Stroke
Last week, Gore REDUCE study, a randomized open-label trial with a median duration of follow-up of 5.0 years [4.8 to 5.2] demonstrated that 1.8% of patients with PFO closure had recurrent ischemic strokes (hazard ratio, 0.31; 95% confidence interval, 0.13 to 0.76), compared with 5.4% patients who treated with an antiplatelet-only group (Figure).1 A patent foramen ovale (PFO) is far and away from the most common congenital heart defect with an estimated prevalence of 1 in 4 adults. The FDA has previously approved the Amplatzer PFO Occluder device in 2016, however initial trials such as the RESPECT, PC, and CLOSER I trials did not show any benefit for PFO closure in the reduction of recurrent embolic stroke, compared to medical therapy. Interestingly, more recent trials conducted within the last 5 years, such as the DEFENSE‐PFO, REDUCE, CLOSE and RESPECT trials, demonstrated that PFO closure had reduced incidence of stroke compared to medical therapy. Given this influx of new evidence from recent trials, it has been suggested that PFO closure be considered in patients 60 years or younger with a PFO-related stroke. However, other potential etiologies such as atrial fibrillation (AF, requires at least 30 days of cardiac monitoring based on recent trials), autoimmune disorders, uncontrolled diabetes or hypertension must first be ruled out.
Last year, the 2020 practice advisory update summary by the American Academy of Neurology suggested that PFO closure probably reduces the risk of stroke recurrence with an HR of 0.41 with acceptable heterogeneity (I2 = 12%) and an absolute risk reduction of 3.4% at 5 years for patients with cryptogenic stroke and presence of a PFO based on meta-analyses using fixed-effect.2 This was unsurprising to me given the trends seen in the RESPECT and CLOSE trials. Interestingly, the report suggested an increased risk of developing AF with RR 3.12 in participants who received closure compared with those receiving medical treatment. This raised an interesting causality dilemma similar to the story of the chicken and the egg. Did these trials capture paroxysmal AF using 30 days of ambulatory monitoring and exclude those with paroxysmal AF prior to PFO closure? If that is the case, what was the primary mechanism for the development of AF after PFO closure? Atrial stunning? If a patient were to develop AF following PFO closure would that increase their risk of recurrent stroke? And if so, is the risk of recurrent stroke higher or lower with PFO closure compared to those without PFO closure? Indeed, it would be interesting see which echo parameters are independent predictors of developing AF in PFO closure (after adjustment for potential confounders). Moreover, the American Academy of Neurology recommends (level C) that aspirin or anticoagulation may be considered in patients who opt to receive medical therapy alone without PFO closure.2 In fact, the comparison between PFO closure and systemic anticoagulation (e.g., DOAC) to prevent recurrent ischemic stroke remains unknown.
Switching gears, let us look at post-PFO closure management. Again, very limited data currently exists on the optimal duration of DAPT (dual antiplatelet therapy) after PFO closure. RESPECT and CLOSE used DAPT for 1 and 3 months, respectively, while some experts recommend ranges DAPT anywhere from 1 to 6 months. A European position paper on the management of PFO, suggested that following PFO closure patients should be on DAPT for 1-6 months followed by antiplatelet monotherapy for ≥5 years.3
In a nutshell, PFO closure should be considered for patients 60 years or younger with PFO-related stroke patients without the comorbidities of the previously mentioned risk factors. A multidisciplinary discussion between neurology, geriatrics, and interventional cardiology are key in decision-making regarding PFO management. Further research should include a randomized controlled trial regarding DAPT duration and the use of DOACs (direct oral anticoagulants) following PFO closure in patients with PFO-related left circulation embolism.
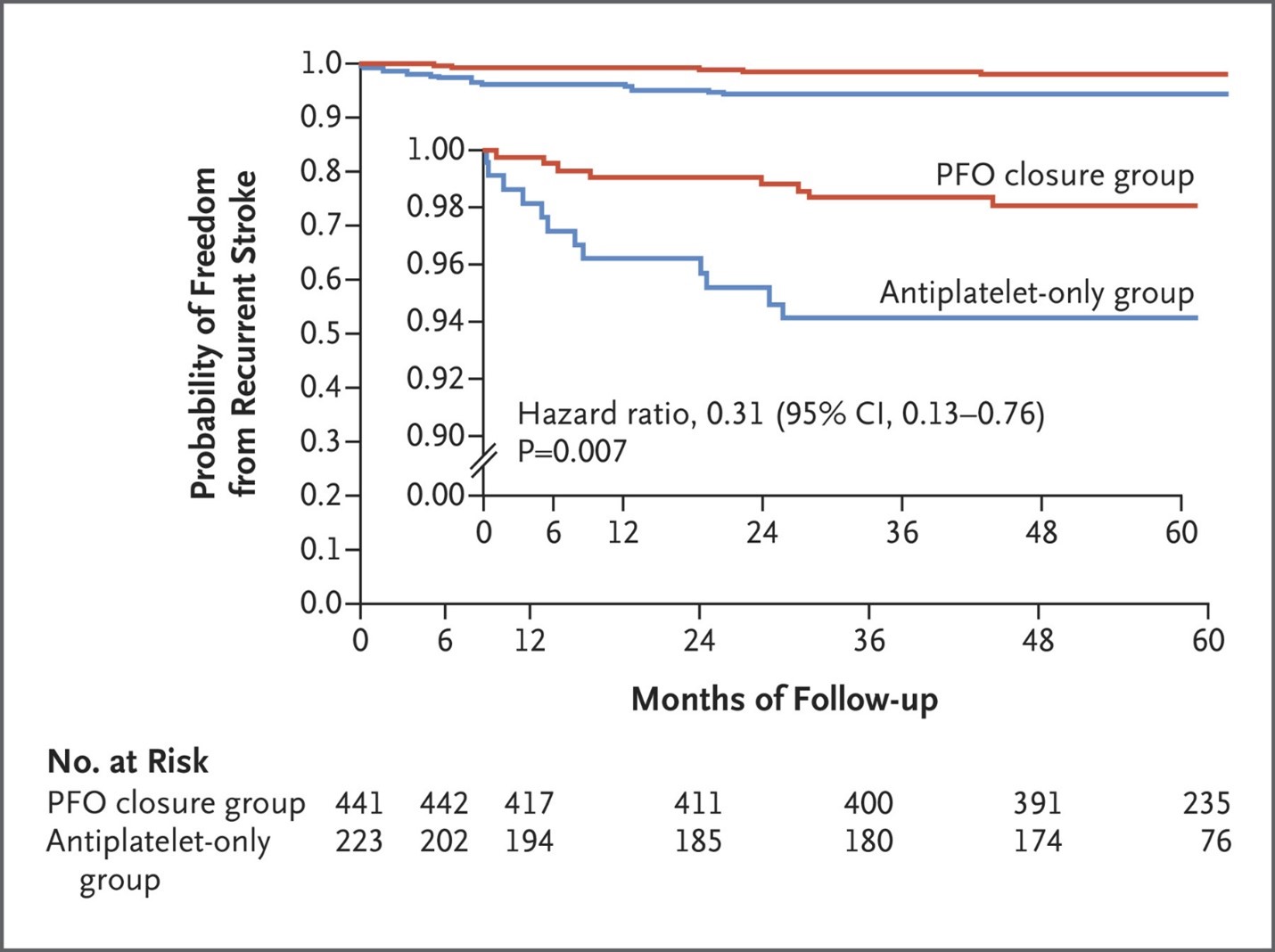
Credit: Figure from the New England Journal of Medicine 2021; 384:970-971
Reference
- Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Søndergaard L; Gore REDUCE Clinical Study Investigators. Five-Year Outcomes of PFO Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med. 2021 Mar 11;384(10):970-971. doi: 10.1056/NEJMc2033779.
- Messé SR, Gronseth GS, Kent DM, Kizer JR, Homma S, Rosterman L, Carroll JD, Ishida K, Sangha N, Kasner SE. Practice advisory update summary: Patent foramen ovale and secondary stroke prevention: Report of the Guideline Subcommittee of the American Academy of Neurology. Neurology. 2020 May 19;94(20):876-885. doi: 10.1212/WNL.0000000000009443. Epub 2020 Apr 29.
- Pristipino C, Sievert H, D’Ascenzo F, Louis Mas J, Meier B, Scacciatella P, Hildick-Smith D, Gaita F, Toni D, Kyrle P, Thomson J, Derumeaux G, Onorato E, Sibbing D, Germonpré P, Berti S, Chessa M, Bedogni F, Dudek D, Hornung M, Zamorano J; Evidence Synthesis Team; Eapci Scientific Documents and Initiatives Committee; International Experts. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur Heart J. 2019 Oct 7;40(38):3182-3195. doi: 10.1093/eurheartj/ehy649.