Transcatheter Interventions of the “Forgotten Valve”
We have witnessed tremendous advances in the transcatheter therapies for various cardiac conditions in the past couple of decades! The “forgotten valve” usually refers to the tricuspid valve, due to the fact that most of the research in the literature is on the left-sided heart valves. In the past two decades, although there has been progressive interest in implementing transcatheter techniques to treat tricuspid valve pathologies, we are still in the early stages of this development. So, I decided to write briefly about some of these transcatheter techniques, which will continue to improve in the future; as we get more experience with these tools and continue to implement the advances in related technologies.
Tricuspid Valve Interventions
There are two main types of transcatheter interventions of the tricuspid valve: repair and replacement.
- Tricuspid Valve Repair
Tricuspid valve repair can involve the tricuspid annulus and/or leaflet coaptation. Leaflet coaptation is performed using the off-label use of MitraClip (Abbott) in the tricuspid valve (also known as TriClip), mainly because of the device availability and operator familiarity [1]. There are other repair systems to repair the tricuspid leaflet coaptation, including Forma (Figure 1) and Pascal systems from Edwards Lifesciences. The Forma repair system consists of a spacer that occupies the regurgitant orifice and thus decrease regurgitation. The Pascal repair system consists of two paddles, clasps and a spacer, thus overcoming some of the limitations of Forma repair system regarding anchoring and dislodgement [1]. Other repair interventions involving the annulus are usually performed using sutures or an annuloplasty ring (Figure 2) [1].
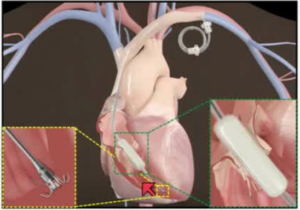
Figure 1: Forma repair system (Edwards Lifesciences), which is a transcatheter approach to improve the leaflet coaptation of native tricuspid valve by occupying the regurgitant orifice area [2].
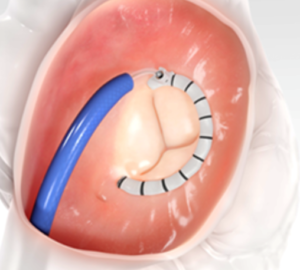
Figure 2: Cardioband (Edwards Lifesciences) is a transcatheter annuloplasty ring for the tricuspid valve [3].
- Tricuspid Valve Replacement
The first transcatheter tricuspid valve replacement was performed by Kefer et al in 2014 using the balloon-expandable SAPIEN valve (Edwards Lifesciences). There are 6-7 types of dedicated transcatheter tricuspid valves that have been developed in the recent years; these include NaviGate (NaviGate Cardiac Structures, Lake Forest, California), Edwards Evoque, Medtronic Intrepid, Lux (Chinese designed and manufactured self-expanding prosthesis made from bovine pericardial tissue mounted on a nitinol stent frame), and Tricares (TRiCares GmbH, München, Germany) valve is a self-expanding prosthesis made from bovine pericardial tissue mounted on a nitinol stent frame. Figure 3 illustrates a NaviGate valve, which is the first tricuspid prosthetic valve implanted in humans in the United States, which was performed by Navia et al in November 2016 [1]. It is an example of a self-expanding dedicated tricuspid valve with 3 pericardial leaflets.

Figure 3: NaviGate valve, which is currently available for transcatheter tricuspid valve replacement [4].
- Caval Stenting
In addition, stenting of the inferior and/or superior vena cava has also been performed to mitigate the effects of tricuspid regurgitation on the central venous system. It is another option for those patients with tricuspid regurgitation, but there are concerns that this procedure might ultimately promote significant hemodynamic deterioration, with ventricularization of the right atrium and increased load on the right ventricle. Ongoing studies are being conducted to assess these effects and the outcomes of this procedure [1].
Do transcatheter interventions of the tricuspid valve affect mortality?
So far, the current evidence we have is from observational studies suggesting improved mortality in patients treated with tricuspid repair/replacement compared to medical therapy [1]. Overall, these transcatheter interventions have shown significant improvement in patient’s symptoms and New York Heart Association (NYHA) functional class, despite only moderate reduction in tricuspid regurgitation severity [1]. There are multiple ongoing trials assessing the impact of these therapies on outcomes. The TRILUMINATE trial is one example, which is comparing outcomes of tricuspid clipping to medical treatment in patients with functional tricuspid regurgitation.
In conclusion, there are several transcatheter therapies that have been developed for treatment of tricuspid valvular pathologies, most commonly for functional tricuspid regurgitation, in the past few years. Although most of these techniques are still in their early stages, the initial results of the observational data are promising. I look forward to seeing the advances in these therapies in the near future, as we continue to build our experience as operators and familiarize ourselves with these new advanced tools.
References
- Lluis Asmarats, Rishi Puri, Azeem Latib, José L. Navia, Josep Rodés-Cabau: Transcatheter Tricuspid Valve Interventions: J Am Coll Cardiol. 2018 Jun, 71 (25) 2935-2956. https://www.onlinejacc.org/content/71/25/2935
- Edwards Transcatheter Approach to Tricuspid Regurgitation – Josep Rodes-Cabau, MD https://rutherfordmedicine.com/videos/Edwards-Transcatheter-Approach-To-Tricusp-FD2C5E468
- Nickenig, G. TRI-REPAIR: 30-Day Outcomes of Transcatheter TV Repair in Patients with Severe Secondary Tricuspid Regurgitation. Presented at TCT 2017 https://www.edwards.com/gb/devices/transcatheter-valve-repair/cardiobandtricuspidsystem
- First U.S. Transcatheter Tricuspid NaviGate Valve Successfully Implanted https://www.dicardiology.com/content/first-us-transcatheter-tricuspid-navigate-valve-successfully-implanted
- TRILUMINATE Study With Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater TR (TRILUMINATE) https://clinicaltrials.gov/ct2/show/NCT03227757
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”