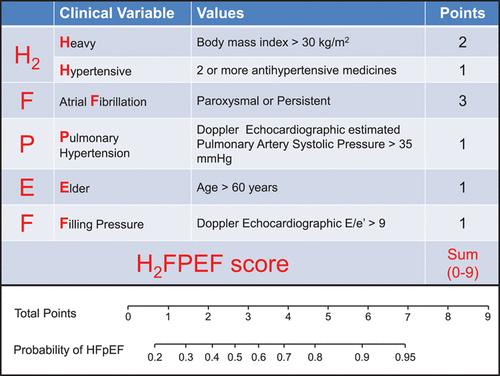
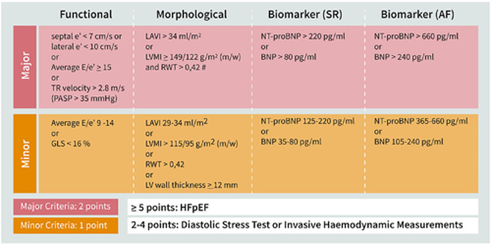
An Interview with Dr. Ilana Kutinsky- Electrophysiologist and Cardiologist of the Apes

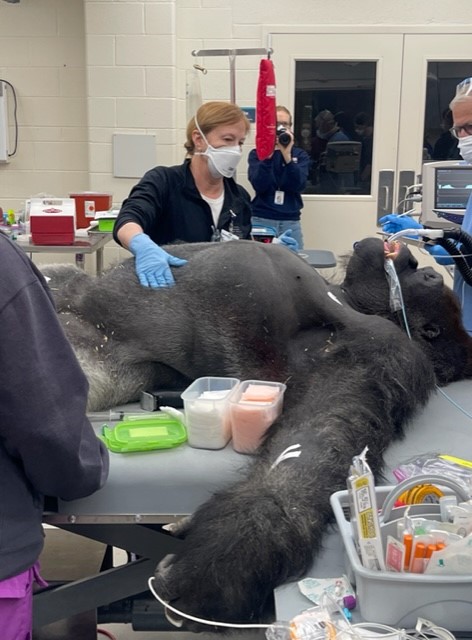
As a resident at Beaumont Hospital, I have the good fortune of working alongside some of the finest cardiologists in the country. I am constantly inspired by talent, innovation, and excellence in patient care. Dr. Ilana Kutinsky, associate professor at Oakland University William Beaumont School of Medicine, takes cardiology and commitment to health equity a step further by providing care to apes throughout the country.
Cardiovascular diseases are the leading cause of mortality not only in humans but also in the great ape species, including gorillas, chimpanzees, orangutans, and bonobos. Established in 2010, the Great Ape Heart Project (GAHP) aims to improve the understanding and management of cardiac disease in our cousin species. A cardiac electrophysiologist by training and profession, Dr. Kutinsky is one of the founders of the Great Ape Heart Project. I sat down with Dr. Kutinsky to learn more about her role as ‘cardiologist of the apes’.
DA: How did you get involved with the Great Ape Heart Project?
Dr. Kutinsky: Around 2000, I was a cardiology fellow at the University of Colorado. During my echo rotation, I picked up a call sitting in the darkroom. “We’re calling from the Denver Zoo. We need someone to come and do TEEs on our gorillas and orangutans. Would you be interested?” and I said “What?”. I talked to my attending and we decided to do it. So, we had Phillips loan us a giant TEE machine and bring it to the zoo. They anesthetized the gorilla and we did the TEE. At the time they were just realizing that captive gorillas die from heart diseases and there was no reference to what is normal. The next month, a female orangutan died from extremis from the anesthesia for the TEE. It was disconcerting to see an animal die for a test we did not even know how to interpret. I got in touch with Dr. Hayley Murphy, who had written a paper reporting echo findings from five gorillas in Boston. We shared our frustration and decided to write a grant to try to collect echos over the country and establish a normal. The grant was rejected but I continued to record readings on my own time by taking freehand measurements on VHS tapes. This is how the Gorilla Heart Project got started. Then it got bigger! We wrote a grant to the Institute of Museum and Library Sciences (IMLS) and got a half a million-dollar grant to set up the Great Ape Heart Project. We were able to hire primatologists, pathologists, veterinarians, and sonographers and set up a database. We have been able to establish normal, identify high-risk animals, and treat heart failure in gorillas. We have consulted on cases not only in the United States but all over the world. It’s a wonderful, although busy hobby for me!
DA: How was your first experience as a cardiologist for the apes, the gorilla in Denver you did the TEE for?
Dr. Kutinsky: It was very similar to taking care of a human once the probe was inside the body. But the animals are enormous, between 350 to 580 pounds. Their chests are like barrels and their skulls are ginormous. To get the TEE probe down their mouths, you have to go past their incisors which are almost the size of my hand.
DA: Were you scared?
Dr. Kutinsky: No, you know they are under anesthesia. And someone is standing in the corner with a big gun. So, if they start to wake up, they will be immobilized. I wasn’t scared because I was so in awe that I was able to do something so amazingly cool. I remember calling my mom afterwards and telling her that every night I spent studying or being on call, all the effort made to get into medical school and cardiology was worth it that day.
DA: Were your co-fellows jealous of you?
Dr. Kutinsky: That’s a good question. Um, I think they thought I was crazy! Doing all that extra work on top of a busy fellowship schedule did not make sense to them.
DA: How is it working with a team of so many disciplines?
Dr. Kutinsky: It is amazing to work with a team of veterinarians, human cardiologists, veterinary cardiologists, sonographers, and pathologists. The vets are honestly some of the smartest people I know. They take care of a 500-pound gorilla with heart disease and then they attend to a tiny bird with an eye injury. Their breadth of knowledge is extraordinary. It has been incredibly rewarding experience working with everyone.
DA: What’s been your most interesting experience being a cardiologist for the apes?
Dr. Kutinsky: Going to Cameroon was one of my most interesting experiences. We were invited by the government of Cameroon to a sanctuary of rescued wild gorillas. We were able to see them up close and interact with them from across a mesh. We were there for 2 weeks, seeing them every day and feeling a connection with them. Usually, I don’t get called when they’re doing well. I’m called when they are sick and about to die, so they’re usually anesthetized. To see them awake, eating and going about their usual life was pretty cool.
DA: Do you have a favorite of the great ape species?
Dr. Kutinsky: Gorillas for sure. I like orangutans too but we don’t have any orangutans in the Detroit zoo so I don’t see them much. Chimpanzees are very naughty and a handful to work with.
DA: Have you become attached to any animal?
Dr. Kutinsky: I was very attached to Sunshine, a gorilla in the Detroit Zoo. He was an old, great animal with a bad heart. He died from influenza a few years ago. I was pretty tight with him. I like Mac in Columbus. He is a very handsome boy. There’s Tatu in Omaha, he’s a good boy. I’ll be sad when they die. Gorillas usually don’t live past 40 years and a lot of the ones with heart disease drop dead early. So we’re hoping to change that.
DA: Do you prefer treating humans or gorillas?
Dr. Kutinsky: Depends on the day! I love animals but I cherish the relationships with my patients. I love my job as an electrophysiologist. Everything I’ve learned as a human doctor I use to treat the gorillas. But if I won the lottery, I would probably devote all my time to the gorillas.
DA: Last question, has taken care of gorillas and other apes affected how you treat your patients?
Dr. Kutinsky: That’s a great question. Hmm, I guess treating animals has made me a more compassionate doctor and person in general. Since the animals can’t complain or whine, you give them symptoms in your mind. You assume they are hurting or sad, and you try to take care of them. I think it has given me an extra bit of sympathy when I take care of my patients. And it makes me happier in general. When you have a passion, it enriches your whole life.
DA: I guess it was a lucky call that day in the echo lab.
Dr. Kutinsky: Absolutely! I was in the right place at the right time. I also think you have to be open to the universe and do things that are outside the box. I was willing to do the extra work outside of a busy fellowship. I was offered something special and I took it.
“The views, opinions, and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness, and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions, or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your health matters. If you think you are having a heart attack, stroke, or another emergency, please call 911 immediately.”