Primordial Prevention of Cardiovascular Disease through Heart-Healthy Diet
The prevalence of cardiovascular disease has been rising in the past decades. Advances in cardiovascular therapies have significantly improved cardiovascular disease survival, which can augment the increasing trends of cardiovascular disease. Thus, the question is raised of how we can prevent this ongoing public health issue. Multiple strategies have been developed to prevent cardiovascular disease, including primordial, primary, secondary, and tertiary prevention. Primordial and primary preventions are established to reduce the prevalence of cardiovascular disease, secondary and tertiary preventions are intended to improve survival and quality of life of patients with cardiovascular disease. Primordial prevention refers to avoiding the development of cardiovascular risk factors― hypertension, diabetes mellitus, dyslipidemia, obesity, smoking, physical inactivity, and poor diet quality― in the first place, while primary prevention is about treating risk factors to prevent cardiovascular disease. Studies have shown that atherosclerosis begins to develop in adolescents. Thus, we must implement prevention strategies early in life. American Heart Association (AHA) introduced the concept of ideal cardiovascular health in 2010 to slow down the rising trends in cardiovascular disease. Ideal cardiovascular health comprises seven components: three factors (total cholesterol, blood pressure, and glucose) and four behaviors (smoking, body mass index, physical activity, and diet). Population-based studies have shown that a meager percentage of the population fulfills all seven components of ideal cardiovascular health. Hence, primordial prevention in early life for the next generation is critical. The brain undergoes maximal developmental plasticity in the first 1,000 days of life. Primordial prevention during this period may place individuals on the healthiest trajectory of lifelong cardiovascular health.
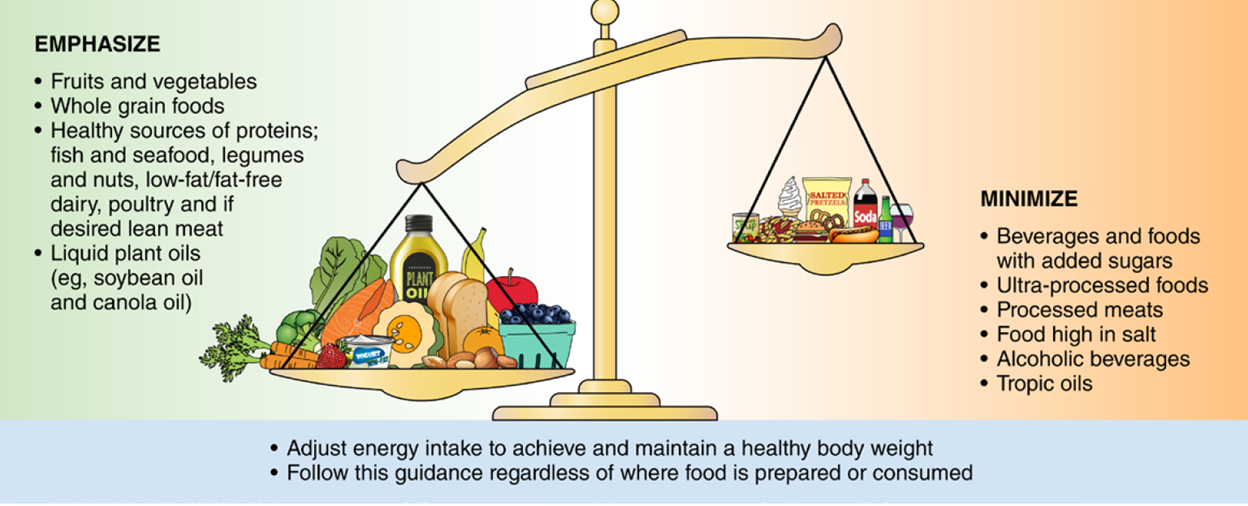
Diet is one of the modifiable components of ideal cardiovascular health. Poor diet quality is strongly associated with elevated risk of cardiovascular disease, and adherence to a healthy diet is crucial in primordial prevention. Last year, the 2021 American Heart Association dietary guideline to improve cardiovascular health was released. The statement highlights the importance of adopting heart-healthy diet habits early in life. Based on the American Heart Association recommendations, a heart-healthy diet contains healthy sources of proteins, mostly plants (legumes and nuts), fish and seafood, low-fat/fat-free dairy, and IF meat or poultry is desired, lean cuts and unprocessed forms were recommended. Moreover, a heart-healthy diet comprises foods made with whole grains rather than refined grains and includes plenty of fruits and vegetables. The statement recommends consuming liquid plant oils such as canola or soybean oil.
Besides selecting healthy food, individuals need to adjust their energy intake to maintain healthy body weight. Body mass index (BMI) is an indicator of general boy fatness. BMI is calculated as body weight (kilogram) divided by height2 (meters). Individuals with BMI ≥ 30 kg/m2 are considered obese, and those with a BMI of 25-30 are overweight. BMI is not a perfect indicator of obesity as it fails to capture fully cardiometabolic risk and is an insufficient marker of abdominal obesity. Other indicators of obesity such as waist circumference or waist to hip ratio may identify patients with abdominal obesity more accurately. Individuals with waist circumference less than 40 inches in men or less than 35 inches in women, and waist to hip ratio less than 0.90 in men and 0.80 in women are considered healthy. Everyone should check their weight routinely and make themselves aware of these numbers to maintain their body weight within these ranges.
The 2021 dietary guideline specifies certain foods we need to minimize consumption. Ultra-processed food is the first one. Ultra-processed is any processed food that, beyond the addition of salt, sweeteners, or fat, includes artificial flavor and preservatives that are added to extend expiration date. Unfortunately, the production and consumption of ultra-processed food are expected to rise through 2024, and society needs to be aware of the adverse risks ―including obesity, diabetes, cardiovascular disease, and all-cause mortality―associated with consuming ultra-processed food. The statement also recommends against food high in salt, tropical oils, and processed meat. Lastly, if someone does not drink alcoholic beverages, it is NOT recommended to start drinking, and those who drink should restrict their alcohol intake to 1 drink per day for women and two drinks per day for men.
“The views, opinions, and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness, and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions, or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your health matters. If you think you are having a heart attack, stroke, or another emergency, please call 911 immediately.”