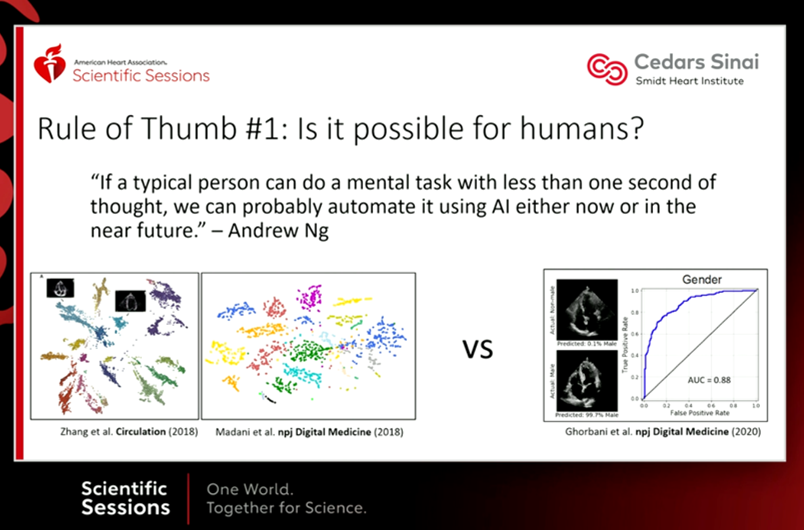
An Experiment with an A.I. Blogging Partner!
This week I wanted to try some new ideas for a blog. Instead of writing it myself, I wanted to test out a new class of artificial intelligence that’s already available, and rapidly expanding in the domain of online media writing. As a blogger, I think it is important to demonstrate what this space is going through in terms of evolution. Therefore, for this monthly blog post, I will be using one of the relatively new Artificial Intelligence (AI) writing generators.
These are applications built on algorithms requiring you to only input a few keywords, or sentences, and then clicking a button and letting the AI generator initiate its programming. The AI will spend some time (only a handful of minutes) and search all over the internet for data, producing a 100% guaranteed unique written article. This type of technology is right now in its infancy stages, and is useful in a limited capacity. But in the next five to ten years, AI of this sort will be immensely more integrated, not just in blogging, but in all types of professional fields.
As a biomedical scientist, I can imagine using an AI generator as an assistant in writing scientific articles that I aim to publish when I generate new research, finalize studies, and have results to share. A medical doctor could use AI writers to assist in composing case reports, as well as discussing details of specific medical treatments, or any other writing task. It’s not hard to imagine a future where many professionals, within academia, or the healthcare system, will be using AI generators to assist them in enhancing, and speeding up content sharing while reducing the time consumption required for writing reports, articles or any other original content.
This is an exciting new technology to embrace as an early career professional. Knowing that my readers (as always, thanks for reading!) are mostly early-career scientists and professionals, I think it’s prudent for me to expose and bring awareness to this emerging technology. With that, I will now share the stage on this blog with the artificial intelligence writing generator called Writesonic. This specific AI generator was picked by me because of its simplicity and affordability. The application only requires me to provide a few keywords, and then provide it time to search and produce a short article that is guaranteed to be unique. There is no “copy-pasting” from somewhere else. And it’s up to me to accept it, and make any changes necessary.
The keywords I provided to seed Writesonic are Heart disease burden novel treatments & prevention strategies. The primer intro I was prompted to provide was this: Heart disease is the number one cause of mortality worldwide. The burden of this disease is set to increase in the coming decades due to longer lifespans, more prevalent sedentary lifestyles, as well as the changing type of diet and food choices that the global population is adapting to. Research towards new preventative measures, as well as new treatment options is of great importance in order to reduce the burden of heart disease on the overall society.
Let’s see what Writesonic produced:
[New Treatments And Prevention Strategies For Heart Disease: A Comprehensive Update
Heart disease is the number one cause of mortality worldwide. The burden of this disease is set to increase in the coming decades due to longer lifespans, more prevalent sedentary lifestyles, as well as the changing type of diet and food choices that the global population is adapting to. Research towards new preventative measures, as well as new treatment options is of great importance in order to reduce the burden of heart disease on the overall society.
What is heart disease?
Heart disease is a term that is used to describe conditions that affect the heart and its chambers (cardiac chamber). Heart disease can be classified as either coronary artery disease or ventricular heart disease. Coronary artery disease is the narrowing of the coronary arteries and the accumulation of plaque, which can lead to a heart attack. Ventricular heart disease is the weakening or malformation of the chambers that give the heart its ability to pump blood, leading to a similar heart attack or a stroke. Approximately 50% of heart disease is due to coronary artery disease. Of this group of diseases, only 50% will progress to cause a heart attack. The remainder are considered non-major conditions, but the symptoms and outcomes can be much worse.
Prevention Strategies
Heart disease is often prevented in its early stages, where the average life expectancy is around 5 years. The first step in the prevention strategies is to prevent a number of risk factors that can cause heart disease, such as high blood pressure, obesity, diabetes, smoking and an unhealthy diet. Some of the proposed strategies for the prevention of heart disease are: Studies show that the common components of heart healthy diets include: vegetables, whole grains, fruit, nuts, low fat dairy products, tea and coffee. Progression to clinical cardiovascular disease can be delayed and even reversed if found in the earlier stages of the disease. Weight gain to obesity may cause a number of health problems, Weight loss is the most important factor in the prevention of heart disease.
Diagnosis and treatment
Heart disease is a major risk factor for death, in both men and women. It is also considered a major cause of morbidity and mortality. Heart disease encompasses all types of conditions in which the heart is significantly damaged. Conventionally, the most common approaches for the treatment of heart disease are drug therapy and surgical intervention. In general, heart disease is caused due to a build-up of atherosclerosis.
Conclusion
Heart disease is a major cause of death worldwide. New therapies and preventative strategies are being developed to reduce the risk of heart disease, as well as new targeted therapies to treat certain forms of the disease. Heart disease is highly complex and the available knowledge and research on the condition is constantly changing.]
Alright, I’m back! Here’s my review: Writesonic is one of the better, but not top of the line, expensive options, so presumably there is a level of competence above what we saw here. The user-friendly and low threshold to entry and use of this AI content generator is very attractive. It allows folks like us, outside of the daily need for such a product, to actually jump in and try this stuff out. The actual end-product, the content, is however way below our “subject matter experts” viewpoints, and for many of us, our “technical precision” will quickly find the overly generalized way the AI writes leaves a lot to be desired.
Without burdening you with another example, I did the same exercise with a different AI writing generator called Rytr, which functions similarly to Writesonic. The final content output was remarkably similar in length and depth of information. This research provided me with enough data to understand where the technology has broadly reached. I see no way for these AI assistants and algorithms to be sufficiently up to date with scientific literature and novel science at this moment. Being able to scan and extract information from original research articles and academic publications is a step (or a mile) out of the general AI writer mandate, for the time being (but surely in a few years this will not be the case).
So, in the end, I’ll say this: AI writers have a great potential to be useful in fields of research and medical writing, but for now, they’re a few years away from that utility. Having said that, for early-career professionals, I say keep an eye on this, you’ll probably be using it by the time you’re at a later stage in the career path you’re on currently!
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”