Optimal Management of Periprocedural Anticoagulation for Catheter Ablation of Atrial Fibrillation
Catheter ablation (CA) of atrial fibrillation (AF) is a safe procedure and the overall complication rates are low. Periprocedural thromboembolic events are one of the most feared complications of this procedure. A large systematic review of 192 studies showed the pooled complication rate of stroke or transient ischemic attacks was only 0.6%1. Despite the low rates of these thromboembolic complications, it is important to explore the factors that contribute to periprocedural thromboembolic events and more importantly ways to prevent them.
It turns out that the periprocedural anticoagulation (AC) strategy has a significant impact on the thromboembolic complications during CA of AF, and the peri-procedural management of AC has been continuously evolving. In the Vitamin K antagonist (VKA) era, the usual practice was to interrupt AC before ablation and then resume it after the procedure with the rationale of minimizing periprocedural bleeding. However, the pendulum moved rapidly after the landmark COMPARE trial. This study enrolled 1584 patients with CHADS2 score ≥1 and assigned them in 1:1 fashion to discontinue VKA or continue VKA during ablation and observed thromboembolic events in the 48 hours after ablation. The study showed that uninterrupted VKA use was associated with a reduction in periprocedural stroke and minor bleeding (odds ratio 13; 95% CI 3.1-55.6; p<0.001)2.
With the advent of direct oral anticoagulants (DOACS) and their improved efficacy in preventing thromboembolic events in patients with AF, an increasing number of patients in clinical practice are on DOACS when they present for CA. Multiple head-to-head trials have shown that uninterrupted DOACS are safe or even better as compared with uninterrupted VKA in preventing procedural thromboembolic events and current guidelines recommend uninterrupted or minimally interrupted DOACS for patients undergoing CA of AF3,4.
Currently, there is wide variability in clinical practice on whether to perform CA with completely uninterrupted DOAC or to omit a single dose or more than one dose? And is there any difference in procedural outcomes between these different strategies?
There is limited data on the comparison of procedural complications with different periprocedural AC strategies with DOACS. Data from randomized trials suggest that there is no difference in thromboembolic and bleeding outcomes whether uninterrupted, single-dose interruption, or more than one dose interruption strategy is used 5. One limitation to this data is that with the low rates of thromboembolic procedural complications in patients taking DOACS, it is hard to demonstrate that one strategy is better than the other. Silent cerebral ischemic lesions are increasingly recognized in patients undergoing CA and it is unclear if in the long term they are associated with dementia or cognitive impairment. An important finding from observational studies is that an uninterrupted DOAC strategy may be preventive against silent cerebral ischemic lesions, however, these results were not observed in randomized trials 6–8.
In summary, a strategy of uninterrupted or minimally interrupted DOACS appears to be safe in reducing periprocedural thromboembolic events for patients undergoing CA.
References
- Gupta Aakriti, Perera Tharani, Ganesan Anand, et al. Complications of Catheter Ablation of Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2013;6(6):1082-1088. doi:10.1161/CIRCEP.113.000768
- Di Biase L, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation. 2014;129(25):2638-2644. doi:10.1161/CIRCULATIONAHA.113.006426
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132. doi:10.1016/j.jacc.2019.01.011
- Romero Jorge, Cerrud-Rodriguez Roberto C., Alviz Isabella, et al. Significant Benefit of Uninterrupted DOACs Versus VKA During Catheter Ablation of Atrial Fibrillation. JACC Clin Electrophysiol. 2019;5(12):1396-1405. doi:10.1016/j.jacep.2019.08.010
- Jafry Ali H, Akhtar Khawaja H, Chaudhary Amna M, et al. Abstract 13721: Is Single Dose Interruption of Direct Oral Anticoagulants Necessary Before Atrial Fibrillation Ablation? A Systematic Review and Meta-analysis. Circulation. 2020;142(Suppl_3):A13721-A13721. doi:10.1161/circ.142.suppl_3.13721
- Müller P, Halbfass P, Szöllösi A, et al. Impact of periprocedural anticoagulation strategy on the incidence of new-onset silent cerebral events after radiofrequency catheter ablation of atrial fibrillation. J Interv Card Electrophysiol Int J Arrhythm Pacing. 2016;46(3):203-211. doi:10.1007/s10840-016-0117-6
- Nakamura K, Naito S, Sasaki T, et al. Uninterrupted vs. interrupted periprocedural direct oral anticoagulants for catheter ablation of atrial fibrillation: a prospective randomized single-center study on post-ablation thrombo-embolic and hemorrhagic events. EP Eur. 2019;21(2):259-267. doi:10.1093/europace/euy148
- Nakamura R, Okishige K, Shigeta T, et al. Clinical comparative study regarding interrupted and uninterrupted dabigatran therapy during perioperative periods of cryoballoon ablation for paroxysmal atrial fibrillation. J Cardiol. 2019;74(2):150-155. doi:10.1016/j.jjcc.2019.02.003
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”

 I was in the mall one day and the saleswoman started talking about her health issues. For starters, I am not sure why she entrusted me with this information, but okay. So why is that conversation interesting enough to write about? Well, the lady was 20 years of age and she had undergone several cardiovascular challenges, including a cardiac ablation. I had heard of other people having this procedure done but I had not thought much about it (I looked it up but not in much detail), until I met this young lady. So, I started wondering:
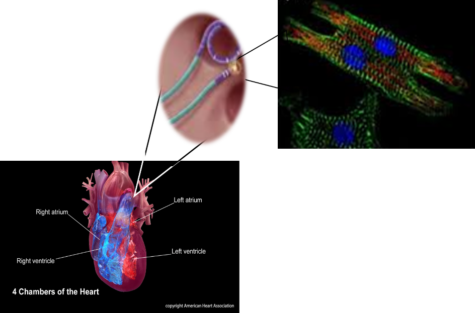
I was in the mall one day and the saleswoman started talking about her health issues. For starters, I am not sure why she entrusted me with this information, but okay. So why is that conversation interesting enough to write about? Well, the lady was 20 years of age and she had undergone several cardiovascular challenges, including a cardiac ablation. I had heard of other people having this procedure done but I had not thought much about it (I looked it up but not in much detail), until I met this young lady. So, I started wondering: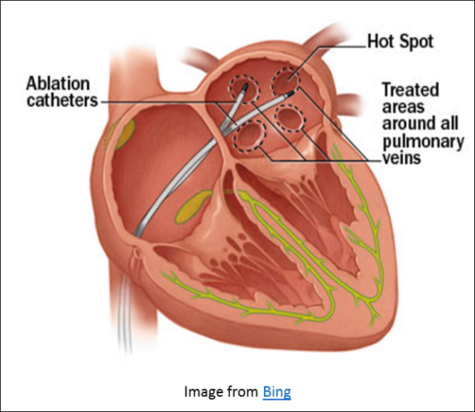 The normal mammalian heart is composed of tight layers of myocytes that are separated by small clefts creating a matrix network. The cardiac matrix network is divided into three constituents. The matrix network is collagen-based and serves as a scaffold for various components of the cell as well as transmission of contractive forces that keep the cells in correct timing with neighboring cells. When the heart undergoes damage, the resulting fibrosis disrupts the coordination of this myocardial excitation-contraction leading to hypertension. Subsequently, loss of collagen impair transduction, which causes the uncoordinated contraction of the cardiac muscle bundles (the quivering or fluttering that is felt with AF) or generation of re-entry circuits (irregular heartbeat).
The normal mammalian heart is composed of tight layers of myocytes that are separated by small clefts creating a matrix network. The cardiac matrix network is divided into three constituents. The matrix network is collagen-based and serves as a scaffold for various components of the cell as well as transmission of contractive forces that keep the cells in correct timing with neighboring cells. When the heart undergoes damage, the resulting fibrosis disrupts the coordination of this myocardial excitation-contraction leading to hypertension. Subsequently, loss of collagen impair transduction, which causes the uncoordinated contraction of the cardiac muscle bundles (the quivering or fluttering that is felt with AF) or generation of re-entry circuits (irregular heartbeat).