Sniffing to Stop SVT
“The aim of medicine is to prevent disease and prolong life, the ideal of medicine is to eliminate the need of a physician.”
William J. Mayo
Paroxysmal supraventricular tachycardia (pSVT) is a common arrhythmic condition known to cause sudden breathlessness, weakness and dizziness due to very rapid heart rates. This syndrome is common in younger patients but can appear at any age. The onset is random, and often without warning. As one can surmise, the symptoms can be debilitating and frightening. Annual emergency room visits for pSVT eclipse 50,000 per year according to Murman and cols [1]. So, what exactly is a pSVT? These arrhythmias are “short circuit” mechanisms which depend primarily on conduction through the AV node and lead to the heart to be trapped in a fast-unrelenting loop. Heart rates of greater than 150-200 beats per minute are common, and the duration can be prolonged (e.g. 30 minutes or longer). Treatment modalities aim to slow conduction through the AV node and either terminate, and prevent the pSVT. The AV Node is a structure at the base of the heart which functions as an electrical delay station of sorts, permitting ventricular diastole. One can consider this the “brakes” of the heart. Although there exist a number of arrhythmias that fall into this broad category, the most common entities are atrioventricular nodal reentry tachycardia (AVNRT) and orthodromic reciprocating tachycardia (ORT). A full description of the anatomy and physiology of these conditions is beyond the scope of this discussion.
Although patients with pSVT have at their disposal prescription agents referred to as “AV Nodal blockers,” these agents serve best as preventive measures. For acute termination, intravenous forms of these drugs can be administered, however this is almost always is in an emergency room or hospital setting. Another of these parenteral agents is adenosine. This is a highly effective drug with a very short half-life but requires hemodynamic monitoring. Given the intensity of the symptoms, affected patients will frequently recur to the emergency room. Many times, these patients are hospitalized. Although some patients are able to terminate an episode by evoking vagal maneuvers (e.g. bearing down etc), most will need medical attention. Ultimately, many of these patients are candidates for catheter ablation, which can be curative. Nevertheless, avoiding a hospitalization is highly desirable.
As I mentioned in my previous blogs, there has been minimal progress in the development of antiarrhythmics. There is one exception, which is a novel agent presented at the Heart Rhythm Society conference of 2017 [2]. Etripamil (Milestone Pharmaceuticals, Montreal St.-Laurent, Quebec, Canada), is the newest form of pSVT treatment. This unique calcium channel blocker has a quick onset and is delivered intranasally. This innovative delivery is attractive as it could lead to a quick resolution of the pSVT episode and avoid a trip to the emergency room. Stambler et al recently published the results of the NODE-1 trial (etripamil in escalating doses vs placebo). The median time to conversion was brisk at less than 3 minutes [3]. Overall, the conversion rates were excellent with the higher doses (See Figure).
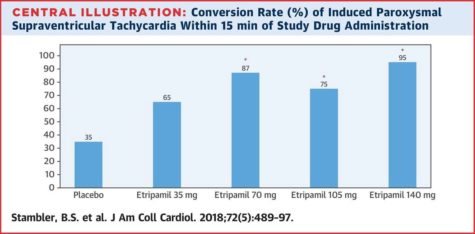
The medication which was tested during a diagnostic electrophysiology study proved to be safe as well; abnormal electrocardiographic changes and hypotension were rarely observed.
If follow-up data continues to be favorable, then etripamil may be of help to pSVT patients in avoiding hospitalization. This agent may have a role for patients who are awaiting ablation, or those whose cases are deemed too high risk for sedation or anesthesia. The use of a simple intranasal route would suggest that nearly everyone would be capable of self-administration.
References
- Murman DH, McDonald AJ, Pelletier AJ, Camargo CA Jr. U.S. emergency department visits for supraventricular tachycardia, 1993-2003.Acad Emerg Med. 2007;14:578-81
- https://www.heartrhythmjournal.com/article/S1547-5271(17)30587-8/pdf
- Stambler BS, Dorian P, Sager PT, et al. Etripamil Nasal Spray for Rapid Conversion of Supraventricular Tachycardia to Sinus Rhythm.J Am Coll Cardiol. 2018;72:489-497
Christian Perzanowski is an electrophysiologist in Tampa, FL. His main interests are in ablation techniques for atrial fibrillation and device therapy for congestive heart failure. He reports no conflicts of interests.
CP (Tampa, FL 7/18)