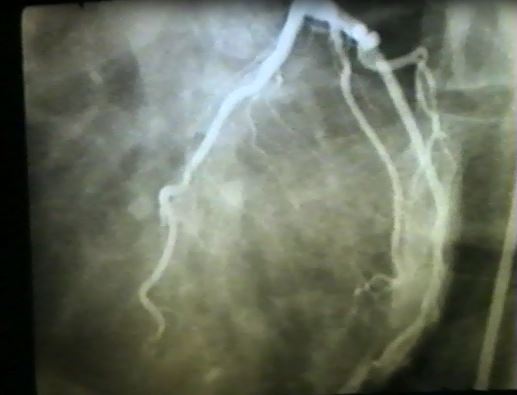
Another (Louder) Call to Improve the Care We Provide Heart Failure Patients
I am always taken aback when I recommend a switch to sacubitril/valsartan in a patient with heart failure with reduced ejection fraction (HFrEF) and the response is “my patient feels fine”. This is a common response and certainly not a good enough reason to not optimize guideline directed medical therapy (GDMT) in patients with HFrEF. … Read more