Inflammation: A Hallmark of Abdominal Aortic Aneurysm Pathophysiology
In my previous blog post, I discussed the animal models used for studying abdominal aortic aneurysms (AAAs), the progressive dilation of the aorta which if left untreated, will lead to fatal aortic rupture. Currently, there are no pharmacological treatments available for this devastating condition and the current clinical approach is to monitor the aortic dimension and eventually, the patient will undergo open or endovascular surgical repair when the aorta attains sufficient expansion. Given the complexity of the disease and the fact that human AAA tissue are acquired at the advanced stages of the disease, researchers have relied on animal models to better understand the disease process and to facilitate development of effective non-invasive therapies.
In general, AAAs are associated with aged population, male gender and lifestyle risk factors such as hypercholesterolemia and smoking. Lessons learned from such studies show that AAA pathological hallmarks include an increased local inflammation in the aortic tissue which can be further augmented by proteolytic degradation of extracellular matrix and depletion of vascular smooth muscle cells.
Inflammation is a common characteristic of AAA, which is manifested by accumulation of inflammatory cells and a wide range of related molecular signaling changes. Macrophages are the most common cell type present in AAA tissue which are localized in media and to a higher extent, in adventitia layer.
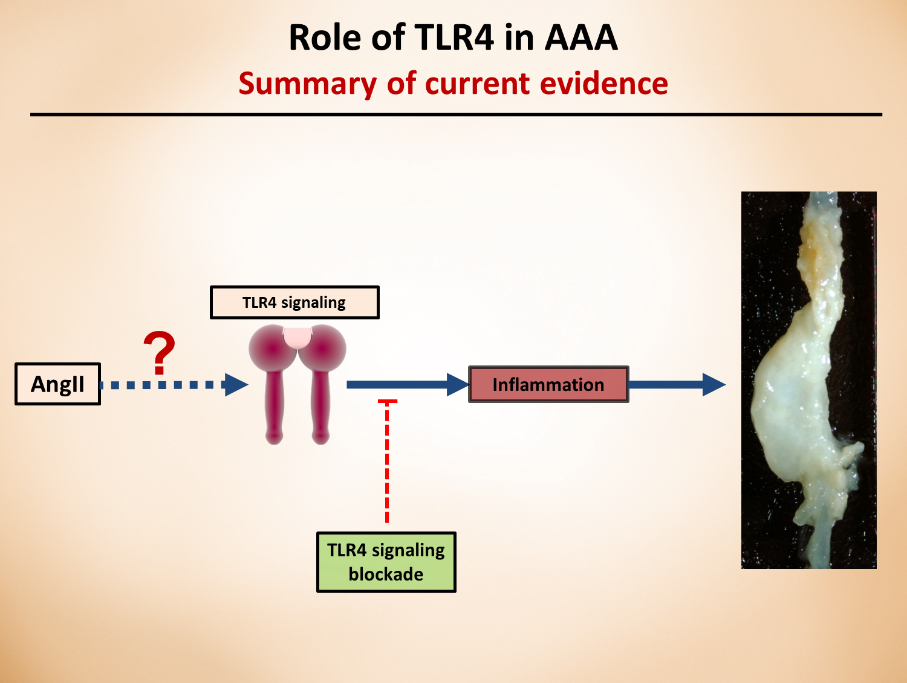
Our laboratory’s previous studies were among the first to show that whole body deficiency of TLR4, a key player in proinflammatory signaling, can ablate the angiotensin II (angII)-induced AAA in low-density lipoprotein receptor deficient (LDLR-/-) mice. Other research endeavors using different mice background or other AAA animal models also further confirmed the critical role of TLR4, or its ligands in AAAs. Although these studies clearly show the role of TLR4 in AAAs, further research is needed to understand which cell types are responsible for TLR4 protective effects and via which mechanisms. As a researcher in this field, my current focus is on targeting aortic cell-specific TLR4 (adventitia), understanding how these cell-specific receptors contribute to the pathology, and the role of potential ligands that activate this cascade of events.
These understandings have the potential to contribute to the development of pharmaceutical approaches that can specifically target the cell receptors and prevent/slow the progression of the pathology.
Resources for Further Reading:
- Renin-Angiotensin System and Cardiovascular Functions: Chia-Hua Wu, Shayan Mohammadmoradi, Jeff Z. Chen, Hisashi Sawada, Alan Daugherty and Hong S. Lu, ATVB, 2018