On February 27, 2021, the Johnson & Johnson COVID-19 Vaccine has been Authorized by the FDA for emergency use. Which makes it the third vaccine to be authorized in the United States. The emergency use authorization was granted after 43,783 participants (18 years of age and older) with no evidence of prior COVID-19 infection were randomized to the vaccine group versus the placebo (saline) group. The trial was conducted in eight countries across three continents with a diverse and broad population. Overall, the vaccine was 66% and 67% effective in preventing moderate to severe/critical COVID-19 occurring after 2 and 4 weeks respectively. Moreover, it provided a 77% and 85% in preventing severe/critical COVID-19 occurring after 2 and 4 weeks respectively. Similar to the other vaccines, the most commonly reported side effects were pain at the injection site, headache, fatigue, muscle aches, and nausea. It is still unclear whether the vaccine will decrease transmission of the virus. Additionally, the participants were only followed up for a median of 8 weeks, so long-term efficiency or safety is not available. One of the main advantages of this vaccine is that it is administered as a single shot.
In contrast to the Pfizer and Moderna vaccines which utilized messenger RNA. Johnson & Johnson’s vaccine used existing technology to add the gene for the COVID-19 spike protein to a modified Adenovirus. After receiving the vaccine, the body will be able to produce the COVID-19 spike protein to trigger the immune system to mount an immune response without causing the disease.
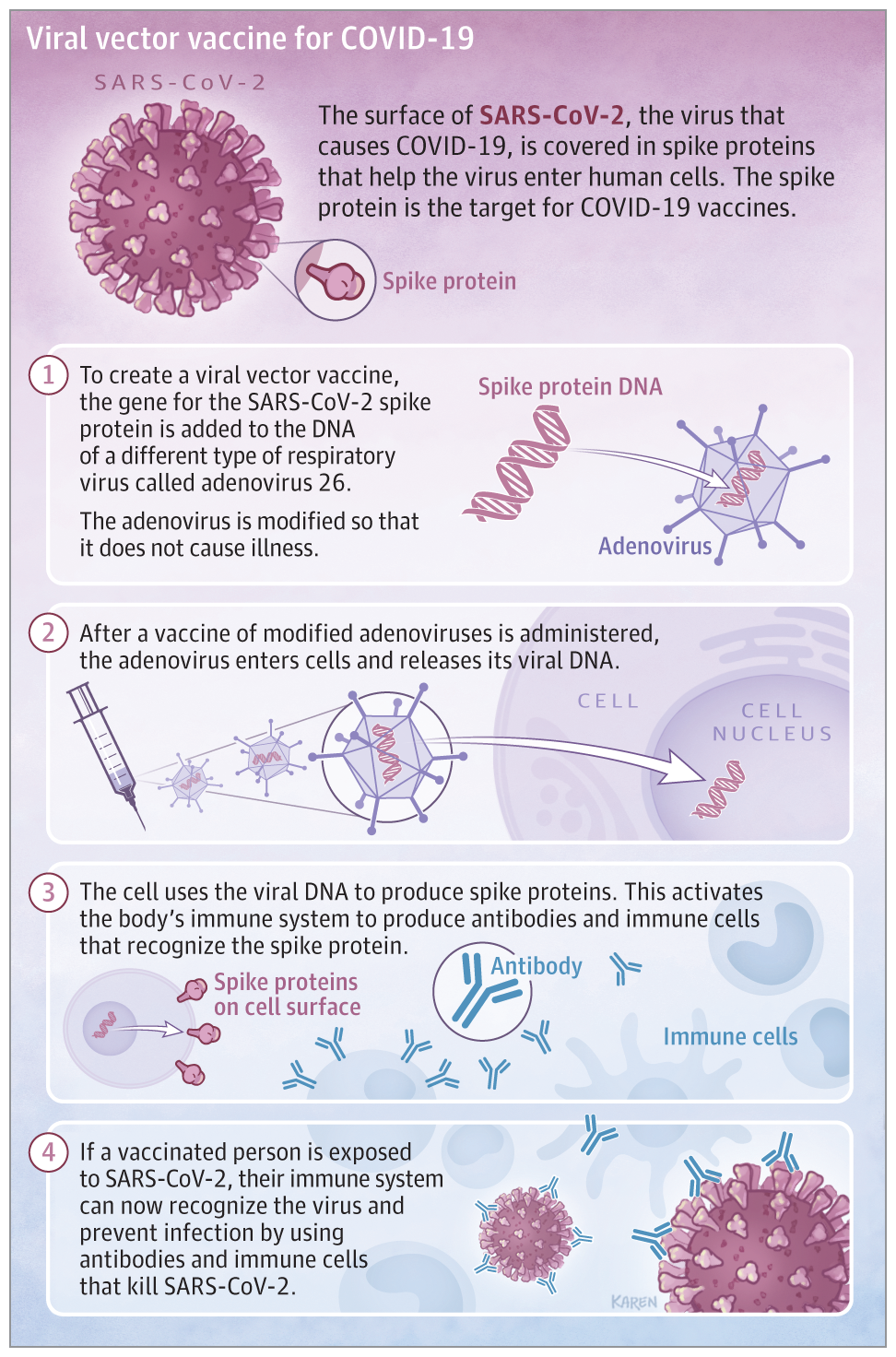
Although the Pfizer and Moderna Vaccines are very effective. Having an additional vaccine will accelerate the vaccination speed. Johnson and Johnson has begun shipping its COVID-19 vaccine and expects to deliver enough single-shot vaccines by the end of March to enable the full vaccination of more than 20 million people. Additionally, Merck will be manufacturing this vaccine which will ramp up the production speed. So far, more than 50 million people have received at least one dose of the vaccine. It is expected that by the end of May 2021, vaccines will be available for the entire adult population in the United States. For the time being, we have to practice social distancing, wear a mask, and hope for the best!
References:
1) Livingston EH, Malani PN, Creech CB. The Johnson & Johnson Vaccine for COVID-19. JAMA. Published online March 01, 2021. doi:10.1001/jama.2021.2927
2)https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
3)https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”