The first two extramural grants for a research project (the precursor to today’s R01) were given on March 21, 1938 to Dr. John Bittner at Jackson Laboratory (then, the Roscoe B. Jackson Memorial Laboratory) in Bar Harbor, Maine for his work on breast cancer1,2.
Bittner J. Mammary Tumors In Mice In Relation to Nursing. Cancer Research. 1937;30.
One grant for $3,200 was awarded directly to Dr. Bittner, and the other for $9,900 was awarded to the Roscoe B. Jackson Memorial Laboratory by the National Cancer Institute. These first “R01-type” awards were given by the NCI from 1938-1943, and in that time, 65 grants-in-aid were paid2.
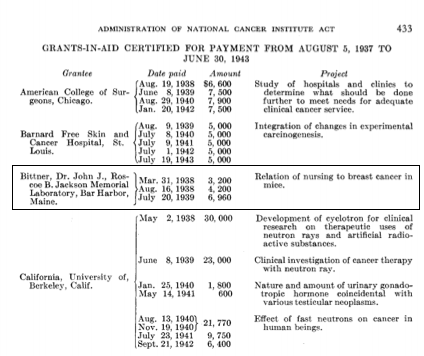
Marshino O. Administration of the National Cancer Institute Act, August 5, 1937, to June 30, 1943. JNCI: Journal of the National Cancer Institute. 1944:429-43.
In the early days of extramural grants in the USA through the 1970’s, most awards were Program Projects, Centers, and Development grants. The NIH preferred to grant awards for multidisciplinary centers to carry out a range of projects in a certain area of interest. It wasn’t until after Watergate and Nixon’s resignation that Investigator-Initiated Research Projects (R01) began to dominate large Program-Project grants (P01)3.
To understand the path from these first grants to today’s R01’s, it is important to understand the history of the NIH. The Hygienic Laboratory became the NIH in 1930 with the passage of the Ransdell Act, and the NCI began with the National Cancer Institute Act in 1937. The NCI could administer extramural grants for research on cancer, but the NIH at large could not3.
The federal government greatly increased research spending in medical research during World War II, and when the war was over, both the government and scientists wanted to continue federally funded research. The NIH couldn’t legally administer extramural grants until the passage of the Public Health Service Act in 1944 which consolidated the NCI and NIH under one roof under the Surgeon General.
In 1946 the Research Grants Office was created to administer extramural research grants and fellowships awards. Congressional funding of the NIH increased steadily, and all the while the NIH was adapting its branding by renaming institutions (i.e. changing “National Microbiological Institute” and “Experimental Biology and Medicine Institute” to flashier names like “National Institute of Arthritis and Metabolic Diseases” and “National Institute of Allergy and Infectious Diseases,” which further enticed congressional funding). From 1946 to 1953, the number of funded projects increased from 80 to 2,000 and the funds increased from $780,000 to over $20,500,0003.
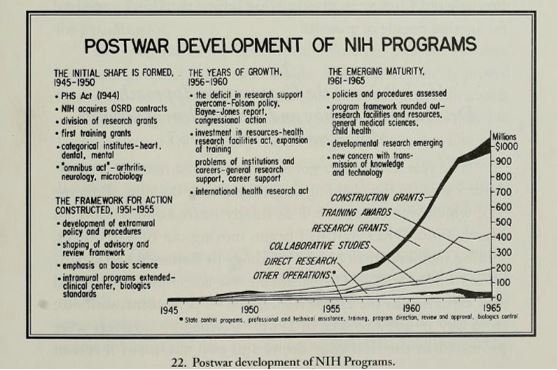
Mandel R. A Half Century of Peer Review, 1946-1996: Division of Research Grants, National Institutes of Health. Division of Research Grants, National Institutes of Health; 1996.
In 1946, the NIH Syphilis Study Section had its inaugural meeting. This was the first study section meeting at the NIH4. From this point, more and more study sections met to discuss the state of science and to review grant applications. In 1950, the standardization of study sections began with the assignment of priority scores from 1 to 5. This made it possible to rank grants based on merit for funding.

Mandel R. A Half Century of Peer Review, 1946-1996: Division of Research Grants, National Institutes of Health. Division of Research Grants, National Institutes of Health; 1996.
These changes lessened the workload of study sections initially, but as research design became more sophisticated, more and more of the meeting time was devoted to project discussions3. Eventually, some within the research community became dissatisfied with the closed-door process of grant review. In 1973, the Office of Management and Budget called for the abolishment of study sections, but the acting NIH Director, Dr. John Sherman, defended peer review as the highest priority.
Political events at the time caused turmoil within the NIH. A combination of budget constraints due to the war in Vietnam along with Watergate and procedural confusion and secrecy regarding the peer review process led to a reorganization at NIH. A familiar name, Ruth Kirschstein, director of the NIGMS in 1974, stated, “How can a system, devised in an era of elitism, of secrecy, and of economic growth…be adopted to an era in which stress in on equal opportunity, openness, and limited availability of funds?”3.

Mandel R. A Half Century of Peer Review, 1946-1996: Division of Research Grants, National Institutes of Health. Division of Research Grants, National Institutes of Health; 1996.
There was rampant favoritism and bias in the review process, and over the next decade, the NIH worked to standardize peer review practices. This included extending project length beyond 3 years in 1984, scoring grants by percentiles in 1987. In the late 1980’s and through the 1990’s, grant applications could be submitted on discs rather than paper and a centralized database of applications was developed.
While the first grant application wasn’t submitted electronically using the PHS-398 form, it was the first time an NIH institute, the NCI, funded an extramural grant for an individual research project, which we now refer to as an R01.
References:
- Bittner J. Mammary Tumors In Mice In Relation to Nursing. Cancer Research. 1937;30(3).
- Marshino O. Administration of the National Cancer Institute Act, August 5, 1937, to June 30, 1943. JNCI: Journal of the National Cancer Institute. 1944:429-43.
- Mandel R. A Half Century of Peer Review, 1946-1996: Division of Research Grants, National Institutes of Health. Division of Research Grants, National Institutes of Health; 1996.
- Pederson T. The “study” role of past National Institutes of Health study sections. Mol Biol Cell. 2012;23(17):3281-4.