If you could peek inside one of the cells in your body, it more or less looks like a factory in which various machines are undergoing different molecular processes. The ultimate goal of this cell machinery is to synthesize a protein. Thus, to produce these complex structures, there are other entities required in the cells known as DNA and RNA.
Double stranded DNA, which holds all the blueprints for the proteins, firstly gets converted into single stranded RNA molecules by the process called transcription and these newly synthesized RNAs are further translated into proteins. In addition to this, there are some negative regulators of the gene expression at the post transcriptional levels known as microRNA (miRNA).
As the name suggests, miRNAs are small 21–25 nucleotide, single stranded, endogenously expressed RNAs which do not code into proteins. These miRNAs are transcribed from DNA in the nucleus and are processed with the help of enzymes (RNA polymerase II and Drosha) and exits the nucleus upon which its further undergoes cleavage with enzyme called dicer. Once matured, miRNA enters the RNA induced silencing complex (RISC) which guides it to target mRNA sites, where it is able to repress protein production by destabilizing the mRNA and induce translational silencing (Figure 1). miRNAs are fine tuners of the gene expression and have been reported to be critical regulators of cardiomyocyte (CM) differentiation, proliferation and other cellular processes during embryogenesis through the adult life.
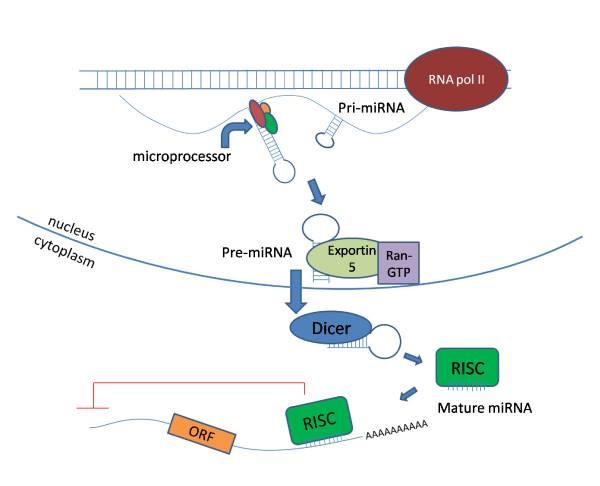
Figure 1: miRNA synthesis and processing in the cell (image taken from reference 1)
So, let’s just talk about role of these powerful miRNA in cardiac regeneration. Many non-mammalian vertebrates are capable of complete regeneration of the heart after an injury throughout their life by re-entry of existing CMs into cell cycle and compensating for the loss of contracting cells. However, mammalian hearts are not blessed with such advantage and CMs undergo nuclear division without cytokines leaving most of the cells binucleated. Under normal conditions, adult mammalian CMs renew to replace the cardiomyocytes that undergo apoptosis at a rate of 0.5–1% a year, while this number is insufficient to replace 1 billion CMs lost during myocardial infarction. Thus, the most feasible strategy to overcome this loss is to coax the preexisting CMs into proliferation.
In the heart, miRNAs have been found to be closely intertwined with cardiac signaling and transcriptional pathways to regulate CM proliferation. High throughput miRNA screening has provided new insights in the role of miRNA in sending the CM back in cell cycle and thus, providing an opportunity of innovative therapies of cardiac repair.
In a recent study, Huang and colleagues reported miRNA-128 upregulation in CMs during the postnatal switch from proliferation to terminal differentiation and hence its deletion extends proliferation of postnatal CMs2. Other experiments conducted in mice indicated the increase in mRNA of 2 targets involved in CM growth and proliferation upon deletion of miRNA -133 family members mi-133a-1/miR-133a-2. miRNA-15 family presents another example for role of miRNA in CM proliferation. miR-195 (member of miR-15 family) was found to be a vital regulator of cell cycle genes and its up regulation inhibits cell cycle progression and induces mitosis arrest postnatally.
Further, studies in zebrafish model demonstrated the downregulation of miR-99 and Let-7a/c miRNA family during of regeneration of amputated ventricular apex while over expression of their target proteins resulted in CM differentiation and proliferation. On the contrary, few miRNA have been identified whose induction have led to remarkable stimulation of CM proliferation and cardiac repair. A study conducted on CMs from adult rats received a lot of appreciation when they showed miR-590 and miR-199a delivery to the cells induced their reentry into cell cycle postnatally3.
The studies mentioned above have shown exciting results which can lead to long-lasting and potent effects of miRNA in cardiac repair especially by the stimulation of post-natal CM proliferation (Figure 2). Despite the successful results obtained in vitro studies, miRNA delivery systems for transporting the molecules to the heart have yet to be optimized.
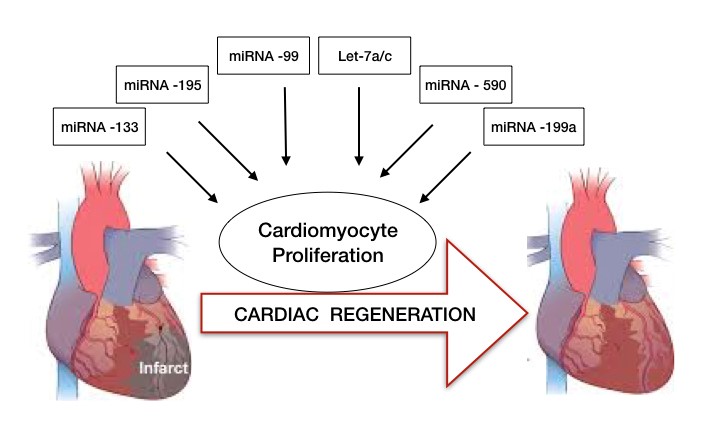
Figure 2: microRNAs in cardiac regeneration
While miRNA is an excellent therapeutic target because of its ability to target multiple genes in a single disease, further works needs to be done to improve its local targeting and reduce cost and toxicity in order to make it possible to use this excellent system to promote CM proliferation in pre-clinical or in clinical settings.
References:
- Beezhold KJ, Castranova V, et al. Microprocessor of microRNAs: regulation and potential for therapeutic intervention. Mol cancer. 2010;9:134
- Huang W, Feng Y, et al. Loss of microRNA-128 promotes cardiomyocyte proliferation and heart regeneration. Nat Commun 2018;9:700.
- Katz MG, Fargnoli AS, et al. The role of microRNAs in cardiac development and regenerative capacity. Am J Physiol Heart Circ Physiol.2016;310(5):H528-41.