Over the past years, series of clinical trials prove the beneficial effect of glucose cotransporter 2 (SGLT2) inhibitors in reducing the risk of cardiovascular events in people with type 2 diabetes mellitus. The results from these trials were consistent, significant, and demonstrated a considerable reduction in heart failure hospitalization among patients who used SGLT2 inhibitors, whereas the benefit on atherothrombotic events such as myocardial infarction and stroke was moderate.
Similar findings from The Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation trial (CREDENCE) were obtained for patients with type 2 diabetes mellitus and chronic kidney disease who are exceptionally at higher risk for cardiovascular disease. In CREDENCE trial, Canagliflozin reduced the risk of chronic kidney disease, cardiovascular death or hospitalization, myocardial infarction, and stroke. Although diabetes is not the only cause of chronic kidney disease, and people with chronic kidney disease are still at increased risk for cardiovascular disease, regardless if they had a preexisting history of cardiovascular disease or not. Therefore, its essential to implement guidelines that recommend the use of certain therapeutics as routine treatment for primary and secondary prevention of cardiovascular disease in patients with chronic kidney disease, regardless of their diabetes status.
During #AHA20, I enjoyed attending the online session by Dr. John McMurray, where he shared scientific breakthrough results from the Dapagliflozin And Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) Mega-Trial. The session reported the results of the effect of dapagliflozin on prespecified kidney and cardiovascular outcomes in patients with chronic kidney disease with and without diabetes. The DAPA-CKD trial was a randomized, double-blind, placebo-controlled, multicenter trial, where adults with or without type 2 diabetes, with estimated glomerular filtration rate (eGFR) between 25 and 75 ml/min/1.73 m2, and a urinary albumin-to-creatinine ratio (UACR) between 200 and 5000 mg/g were eligible for DAPA-CKD trial. In this trial, patients were randomized to dapagliflozin 10 mg once daily or placebo with follow up at 2 weeks, 2,4, and 8 months and at 4 months intervals thereafter. The primary composite outcome was the time to the first occurrence of any of the following: > 50% decline in eGFR, onset of end-stage renal disease, or death from kidney or cardiovascular disease. Moreover, secondary outcomes were: 1) kidney composite outcome identical to the primary endpoint with the exception of death from cardiovascular death 2)( a cardiovascular composite outcome consisting of hospitalization for heart failure or death from cardiovascular causes; and 3) death from any cause.
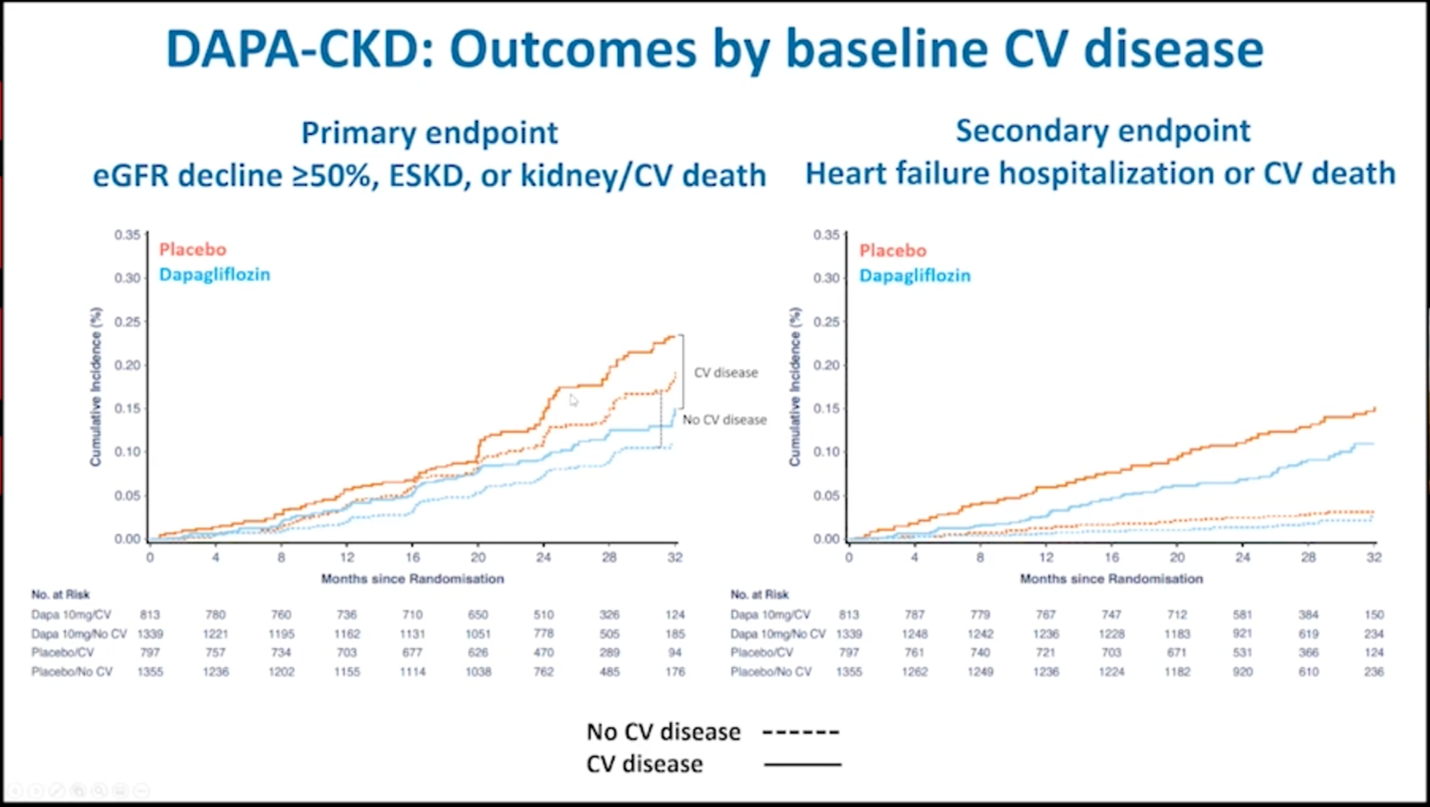
Effects of dapagliflozin on prespecified clinical outcomes according to the baseline history of cardiovascular disease.
The DAPA-CKD trial found that among patients with cardiovascular disease who received dapagliflozin, the primary composite outcome occurred in 11.2% participants, while the primary outcome occurred in 17.2% in participants who were in the placebo group, (HR 0.61; 95% CI, 0.47-0.79) and the corresponding numbers in people without cardiovascular disease were 7.9% and 12.9% respectively, (HR 0.61; 0.48-0.78).
The DAPA-CKD trial also found that for both the primary and secondary prevention patients, the event rates favored dapagliflozin for all components of the primary and secondary outcomes, although reduction in cardiovascular risk was not statistically significant.
DAPA-CKD Figure
Additionally, among patients with cardiovascular disease, cardiovascular death or hospitalization for heart failure occurred in 9.3% of participants in the dapagliflozin group and 12.8% of participants in the placebo group, (HR 0.7; 0.52-0.94) and the corresponding numbers for patients without cardiovascular disease were 1.8% and 2.7% respectively, (HR 0.67; 0.40-1.13). The observed reduction in cardiovascular risk for these two groups was driven by reduction in heart failure hospitalization which occurred in 4.1% of participants in the dapagliflozin group and 7.3% participants in the placebo group with cardiovascular disease and the corresponding numbers for patients without cardiovascular disease were 0.3% and 1.0% (HR, 0.31; 0.10-0.94) respectively. These results show that dapagliflozin reduced the risk of adverse kidney outcomes irrespective of baseline cardiovascular disease status. Moreover, the mortality benefit from dapagliflozin as demonstrated from the DAPA-CKD study supports the findings of the DAPA-HF trial. In summary, dapagliflozin reduced the risk of kidney failure, death from cardiac disease or hospitalization for heart failure, furthermore, it prolonged survival, in people with chronic kidney disease, irrespective of the presence of a concomitant cardiovascular disease.
What is next?
The data from DAPA-CKD trial for dapagliflozin effect on patients with cardiovascular disease and chronic kidney disease is clear, but we have so much work to do. Is Dapagliflozin the answer? How would this change the guideline directed medical therapy (GDMT) for the care of patients with an increased heart failure, cardiovascular or chronic kidney disease risk, regardless of their glycemic status?
References:
- Effect of Dapagliflozin on Clinical Outcomes in Patients with Chronic Kidney Disease, With and Without Cardiovascular Disease. John J.V. McMurray , David C. Wheeler , Bergur V. Stefánsson , Niels Jongs , Douwe Postmus , Ricardo Correa-Rotter , Glenn M. Chertow , Tom Greene , Claes Held , Fan Fan Hou , Johannes F.E. Mann , Peter Rossing , C. David Sjöström , Robert D. Toto , Anna Maria Langkilde , and Hiddo J.L. Heerspink for the DAPA-CKD Trial Committees and Investigators
- Presented by Dr. John J. V. McMurray at the American Heart Association Virtual Scientific Sessions, November 13, 2020.
- Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al., on behalf of the DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients With Chronic Kidney Disease.N Engl J Med 2020;383:1436-46.
- Presented by Dr. Hiddo J.L. Heerspink at the European Society of Cardiology Virtual Congress, August 30, 2020.
- Rationale and protocol:Heerspink HJ, Stefansson BV, Chertow GM, et al., on behalf of the DAPA-CKD Investigators. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020;35:274-82.
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”