Both left and right atrium modulates ventricular filling through three components: a phase of reservoir or expansion during ventricular systole, a conduit phase during diastole, and an active contractile component (when sinus rhythm is present) during late diastole. During exercise atrial reservoir and booster functions are augmented, whereas conduit function is not; increased reservoir function may play an important role in accelerating ventricular filling by helping to maintain an enhanced atrioventricular pressure gradient during diastole and also by increasing atrial booster function through an increase in preload.
The resurgence of interest in atrial size and function has enhanced our understanding of the atrial contributions to cardiovascular performance in health and disease. Considering the limitations of conventional echocardiographic techniques for atrial functional assessment, the high feasibility and reproducibility of bi-atrial strain, and its predictive value in several clinical conditions, biatrial measured by 2D speckle tracking echocardiography could be considered to assess atrial function in the clinical routine.
Methodology of biatrial strain and strain rate: Key measurements and guidance
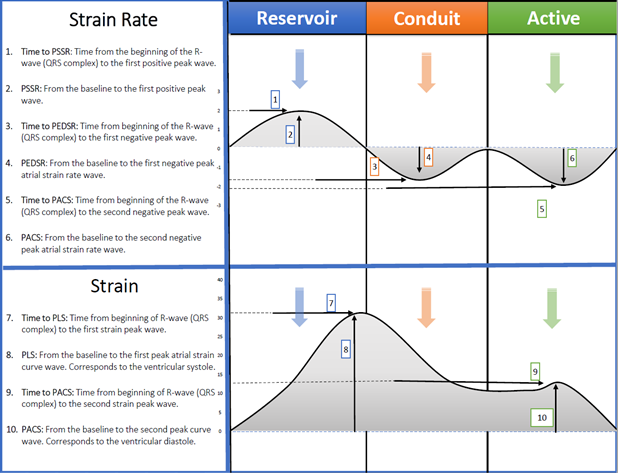
Figure 1: Fawaz Alenezi – Methodology of biatrial measurements 2013: 1. Time to Peak systolic strain rate [PSSR], 2. Peak systolic strain rate [PSSR], 3. Time to peak diastolic strain rate [PEDSR], 4. Peak diastolic strain rate [PEDSR], 5. Time to peak contraction strain rate [PCSR], 6. Peak contraction strain rate [PCSR], 7. Peak longitudinal strain [PLS], 8. Time to peak longitudinal strain [PLS], 9. Time to peak active contraction strain [PACS], 10. Peak active contraction strain [PACS].
Interpretation of atrial functional indices is complicated because of the interplay between atrial and ventricular functions. Atrial dysfunction may result from an intrinsic atrial abnormality, altered load, or in an effort to compensate. Atrial dysfunction may also have different expressions at different stages of the disease process under study. The methods used to measure atrial function all have important limitations and indices from one method (e.g. volumetric) that reflect a specific atrial function often correlate poorly with other methods (e.g. tissue tracking) obtained during the same phase of the cardiac cycle. Finally, the hemodynamic and biophysical properties that are responsible for the functional changes are often assumed, not known.
Technical factors that may influence biatrial strain values:
- Image quality: Optimization of images quality and frame rates are vital (ideally, no less than 40 fps) determinant of accurate edge detection, tracking, and strain assessment.
- Technical differences: A number of different vendors offer strain platforms with technical differences among proprietary post-processing algorithms. There are vendor-specific differences among how overall reported atrial strain values are calculated.
- Technical issues and trackability: Difficulties tracking atrial segments relate to the thin wall, insertion of pulmonary veins, or caval veins, the aorto-atrial or pulmono-atrial curtain (in the three-chamber views), and the lack of dedicated tracking algorithms for atrial strain assessment. Indeed, until very recently, the approach to atrial strain involved ‘‘tricking’’ the LV strain computation algorithm which have different values that a dedicated atrial software’s.
Operator experience: The results may dependent on the individual studies that comprise the analysis and level of expertise are rarely documented. - Zero reference point: If the ventricular cycle is used, ventricular end-diastole (the QRS complex) is the zero reference, and the peak positive longitudinal strain corresponds to atrial reservoir function and the strain during early and late diastole correspond to conduit and atrial booster function. If the atrial cycle is used, atrial end-diastole (onset of P wave) is the zero reference, and the first negative peak strain represents the atrial booster pump function, the positive peak strain corresponds to conduit function, and their sum represents reservoir function.
- Regional and methodological differences in atrial strain: Previous studies have described differences in mean strain ranges between two-chamber (37.6%–44.3%) and four-chamber (33.8%– 40.1%) strain. However, and again this all depend on (image quality and acquisition; software used; where to measure (endocardial, myocardial, median); and post-processing of data.
- Age and sex differences: Significant age-related reductions in deformation have been reported. Similarly, sex-related differences have been described, with lower deformation noted in male patients than in female patients across all age groups studied.
- Hemodynamics factors: Atrial strain increases in response to early physiological heart rate increase in the setting of exercise in normal patients. However, decreased values are found in the setting of pathological heart rate increase, most notably in sepsis.
There is now a growing body of literature supporting its use in different clinical settings. I believe biatrial strain is ready for clinical practice. Its reproducible and has been shown to add unique data that can guide diagnosis and management. I recommend atrial strain as a valuable complement to traditional function parameters. Further studies are needed to standardize vendors, recognizing specific strain patterns and to determine if there are age, gender variabilities or loading conditions difference.

Dr. Fawaz Abdulaziz M Alenezi is a Clinical Imaging Fellow at the Duke University Health Systems. He conducts medical research on the derivation and validation of novel echocardiographic approaches to myocardial deformation and a new echocardiographic technique which assists patients with heart ventricular function.