Gut Microbiota Modulation: From Bench to Bedside
In a series of previous blog posts, I delved into the role of the gut microbiome and its contribution to cardiovascular health. As it is almost time to wrap up this year’s blogging series, I thought to provide some final points about this topic.
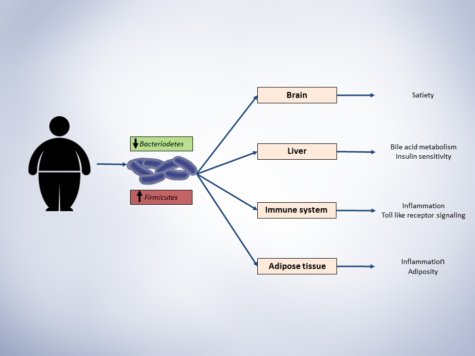
Large lines of evidence that shows gut microbiota is a major player in host metabolism homeostasis, has led to increased interests in leveraging findings for therapeutic aims in cardiometabolic complications. Here, I propose a framework for modulation of gut microbiota with therapeutic purposes (figure):
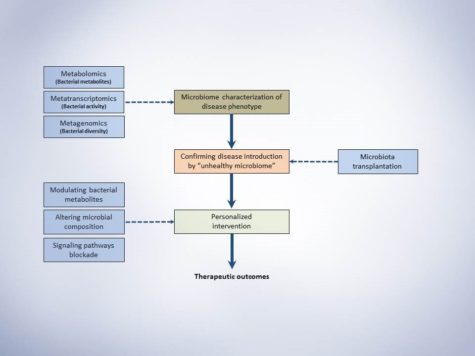
Schematic presentation of microbiota study frame-work. This simple representation suggests three major steps for conducting a microbiome study with the aim of investigating a disease phenotype and possible therapeutic outcome.
1. The first step would be characterizing the microbiome of disease phenotypes illustrating the alterations of specific bacterial taxa and metabolites.
2. Secondly, Koch’s postulation should be fulfilled. In short, the specific taxa should be found in abundance (or indicate specific ratio/levels) in the organism with disease phenotype but not in the healthy phenotype. Secondly, the responsible taxa (or the specific ratio/level of abundance) should be isolatable (or reproducible) and finally, transfer the disease-related taxa (or creating specific ratio/level responsible for disease) to the healthy host microbiome should introduce the disease.
3. After identifying the responsible bacteria or produced metabolites that fulfilled the Koch’s postulation, the third step would be designing an intervention based on the cardiometabolic complication, its progression level and personalization of intervention for each patient. Approaches to therapeutically modulate gut microbiota would be using probiotics, prebiotics, dietary constituents and drugging the microbiome for more specific targeting. Jamming microbiota communication, microbiome programming with modified smart bacteria and the introduction of RNA-guided nuclease CRISPR using bacteriophage carrier are among the new approaches that are starting to form for modulation of the gut microbial endocrine organ. Moreover, fecal microbiota transplantation (FMT) is also among the new approaches in treating metabolic anomalies and recently initiated clinical trial “Fecal microbiota transplant for obesity and metabolism” (ClinicalTrials.gov NCT02530385) is expected to show interesting results in the near future. Still, as mentioned throughout the series of blog posts, our understanding from complex interactions and functions of gut microbiome is in infancy and further animal and human studies are required to shed light on precise microbial targets and prevent the unforeseen consequences of long-term microbial disruption.
Conclusion and Closing Thoughts
Indeed, the community of bacteria residing in the human body was ignored for many years. But, recent evidence started to shape the idea that human’s microbial symbionts play multiple functional roles in maintaining normal metabolic functions. Successful improvement of metabolic syndrome and obesity that was discussed throughout these blog series indicate that future treatments may be, at least partially, based on microbiota interventions. More precise interventions should be developed to address the desired modulatory effect, yet, it raises new challenges since a major portion of gut bacteria is still uncultured. Also, regulatory aspects of current interventions including FMT, probiotics, prebiotics and bacterial metabolite inhibitors should be addressed in more detail since neither formulations development nor quality control guidelines are available. Moreover, it is time to move forward from small cross-sectional studies to more large-scale epidemiological investigations to understand better whether the microbial alterations cause disease development or the complications itself results in such alterations. In order to make the results of small and large microbial studies more clinically implantable, the field needs to generate universal standards for sample collection, data analysis, and sequencing to allow reproducibility and unbiased comparisons between different studies.
Despite all the hurdles in microbiome studies and translation of findings, there has been a bloom in researchers and companies looking for diagnostic and therapeutic strains and approaches to modulate them and track such modulations. The latter is more emphasized by the recent announcement from White House Office of Science and Technology Policy for the launch of United States National Microbiome Initiative (NMI) that aims to foster microbiome studies in different ecosystems. NMI aims to expand the microbiome workforce, develop platform technologies and support research to advance our understanding of microbiome and restoring its healthy function in different complications.
In the end, it is vivid that this novel area of research may impact medicine in the very near future and by addressing the current challenges, incorporated microbiome-based diagnostic and therapeutic protocols into patient care starts to emerge.

Shayan is a caffeine-dependent Ph.D. Candidate at the Saha Cardiovascular Research Center, University of Kentucky. His research area is focused on vascular biology and lipid metabolism. He tweets @MoradiShayan, blogs at shayanmoradi.com and he is the Winner of World’s Best Husband Award (Category: nagging).