Live Streaming Into Scientific Sessions 2018
AHA Scientific Sessions 2018 was a unique experience for me – unable to attend the meeting, I live-streamed the sessions (first time ever for a conference!). Two of my most favorite sessions this year were the panel discussion for advanced heart failure (HF) patients, “The Metabolic Face of Heart Failure,” and the mini-symposium on “Cutting Edge in Cardiovascular Science.”
One of the main highlights in the session Metabolic Face of HF, moderated by Dr Lynne Stevenson, was the talk by cardiovascular stalwart Dr. E. Braunwald, Brigham and Women’s Hospital. Dr. Braunwald spoke of the significance and latest practices in the use of Sodium-Glucose Cotransporter-2 (SGLT2) inhibitors, a class of FDA-approved drugs for type-2 diabetes. He indicated how SGLT2 inhibitors should be explored beyond diabetes treatment and these class of drugs can benefit HF patients as well. “I had to learn about blood clotting 30 years ago, which was difficult,” he modestly admitted as he clarified the renal effects of SGLT2 inhibition. His views also seemed to resonate with Dr. Subodh Verma, St. Michael’s Hospital, Toronto and Dr. John McMurray, Glasglow University, as they covered SGLT2 inhibitors in HF, as well.
Other speakers at this session, Dr. Neha Pagidipati, Duke University and Dr. Lewandowski, Ohio State University, touched upon aspects of stroke and metabolism regulating HF, respectively. While Dr. Pagidipati compared the risk of cardiovascular diseases and stroke with the risks of diabetes, Dr. Lewandowski explained how metabolic regulator PPAR-a (transcriber of genes in fat metabolism) could be a player explored in targeted therapy.
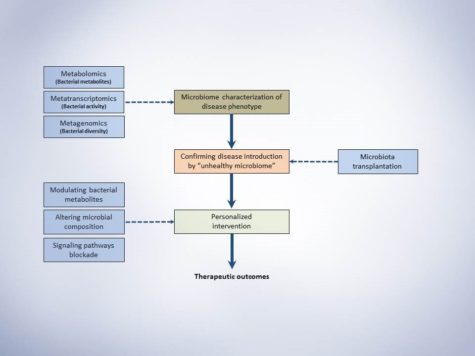
The session ‘Cutting Edge in Cardiovascular Science’ had presenters covering diverse strategies in dealing with cardiovascular therapy, ranging from computational screening to identifying small molecule compounds, to decoding neurovascular networks and the gut microbiome. Dr. Stanley Hazen from Cleveland Clinic presented his work on understanding the microbes in the gut and their role in driving cardiovascular diseases. Dr. Hazen explained how food like red meat, which are rich in components like phosphatidyl serine, activates the gut microbiome. He described the significance of trimethylamine N-oxide (TMAO) pathway in liver and its association with HF, stroke and cardiovascular diseases. He also strategized the use of enzyme in TMAO pathway as targets of small molecule inhibitors.
Dr. Joseph Loscalzo, Brigham and Women’s Hospital, explained how repurposing drugs and finding drug targets computationally could help precision medicine vastly. He also offered his expertise and tools as open access to AHA members. Finally, Dr. Costantino Iadecola, Cornell, elaborated on the heart-brain connectome. He brought attention to the fact that dementia, known to cause hardening of arteries, led to Alzheimer’s, but we all forgot about the vascular complications of this. He bridged this connection between neurovascular dysfunction and cognitive impairment and went on to explain his research on the intake of high salt in diet caused dementia in mice models. To learn of such versatile range of topics in a session was illuminating, to say the least!
Researchers must spend time thinking about applications of their current projects beyond their own niche – this is the only way we can widen our horizons with existing tools.