Clinical practice guidelines find extensive usage worldwide amongst both researchers and clinicians. In 2021, I had the opportunity to act as one of the trainee members of two guideline groups of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) with the experience being transformative. The following will be a multi-part series on the why’s and how’s of guidelines, getting behind the scenes on their development.
Need for Clinical Practice Guidelines
The American Heart Association (AHA) and the American Stroke Association (ASA), often in conjunction with the American College of Cardiology, produce major clinical practice guidelines (CPGs) that are both widely utilized as professional practice standards globally and used extensively for research. For instance, the guideline papers on ‘Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults’ by the AHA/ACC Task Force on CPGs, which were published in multiple journals simultaneously, have been cited thousands of times by the end of 2021 since their publication in 2017, while having been utilized by millions of clinicians worldwide [1].
These guidelines are critically valuable tools for practitioners, many of whom lack the time to appraise the primary literature themselves. The latter becomes an especially challenging endeavor given the increasing amount of publications along with the expanding number of low-quality studies. In her book, Trisha Greenhalgh had quoted Stephen Lock, who had described in 1979 as the editor of the British Medical Journal, “Few things are more dispiriting to a medical editor than having to reject a paper based on a good idea but with irremediable flaws in the methods used” [2]. In 1994, Douglas Altman, one of the pioneers of the EBM movement, had written those widely quoted words that “We need less research, better research, and research done for the right reasons” [3]. A decade later, John Ioannidis had published his landmark work ‘Why most published research findings are false’ [4].
Twenty years after Altman’s editorial in BMJ, the editor of BMJ wrote how little had changed since then [5]. Chalmers and Glasziou have classically described the four stages of research waste [6], which coupled with the predicted doubling time of medical knowledge reaching 73 days in 2020 [7], leads to the increasing difficulty of reading and synthesizing the primary evidence for oneself. Thus, in an age of information overload, appropriately developed and concisely written guidelines in one part of the world often become a critically valuable tool globally.
Guidelines also help define the value judgments we assign to the primary evidence. For instance, for acute management of ischemic stroke, different anticoagulating drugs may be drugs, with one negative outcome assessed in the literature being the risk of intracranial hemorrhage. Here one value judgment is the significance we assign to the occurrence of intracranial hemorrhage. Finally, for outcomes where high-quality primary evidence is lacking, guidelines may still provide conditional recommendations with low certainty, which are useful to the frontline healthcare worker.
Evolution of Clinical Guidelines
Guideline development has come a long way from before the advent of the evidence-based medicine (EBM) movement to after the widespread utilization of the same. Colloquially, the old approach to guideline development was the GOBSAT model, i.e. ‘Good Old Boys Sitting Around A Table’ [8,9]. This typically was based on a panel of experts (typically males) who would be invited by a professional society to convene for a few days. They would provide their opinion and discuss their clinical practice, and their consensus would get written up as the professional society guideline to be followed worldwide. Many times, this gathering would occur in a hotel or trip funded by a pharmaceutical corporation [9].
In addition, this approach majorly ignored conflicts of interest amongst the experts themselves, since no rules existed on who could participate as a panel member based on their competing interests. Experts, who were paid speaking fees or honoraria related to certain medications by pharmaceutical corporations, would overwhelmingly represent members of the task force making the guidelines on diseases where those drugs would get utilized [9]. These competing interests meant that guideline development had significant bias from the start.
Finally, even if subject matter experts did not have competing interests, it was still likely that their perspective of the literature in totality on the PICO was biased, as was well captured in seminal papers by Mulrow in BMJ and Antman et al in JAMA [10,11]. These authors demonstrated that traditional narrative review-based consensus opinions of experts greatly lagged behind and sometimes significantly differed from effect estimates from well-performed meta-analyses. In one of their classic examples, Antman et al used the process of cumulative meta-analysis to show that data was consistently building up regarding the futility of lidocaine in improving survival in acute myocardial infarction for many years, yet most major traditional reviews continued to wholeheartedly recommend this medication even after 1990 [11].
Current Model of Guideline Development: An Overview
One of the most widely cited definitions for CPGs or ‘guidelines’ was given by an influential Institute of Medicine (IOM) report which referred to them as “statements that include recommendations, intended to optimize patient care, that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options” [12]. This definition can be broken down into two parts: (A) a systematic review of the primary evidence, which is the groundwork of the guideline, and (B) a set of recommendations, which incorporates both the evidence and the value judgments. The systematic review is typically informed by one of more key questions, that are structured in the PICO format, i.e. Population, Intervention, Comparator, Outcome(s).
Guidelines today are developed by a multidisciplinary panel of experts, which include but are not limited to, subject matter experts (which were the only members of guidelines previously), guideline methodologists, health economists, statisticians, librarians, patient representatives, amongst others (Figure 1). The goal now is to have guidelines that focus on what matters to the patients, and therefore, the outcomes used for decision-making in the guidelines have shifted gradually towards patient-reported outcomes (PROs). Instead of conventional outcomes like blood loss, PROs include pain score, quality of life, time to return to activities of daily living, etc.
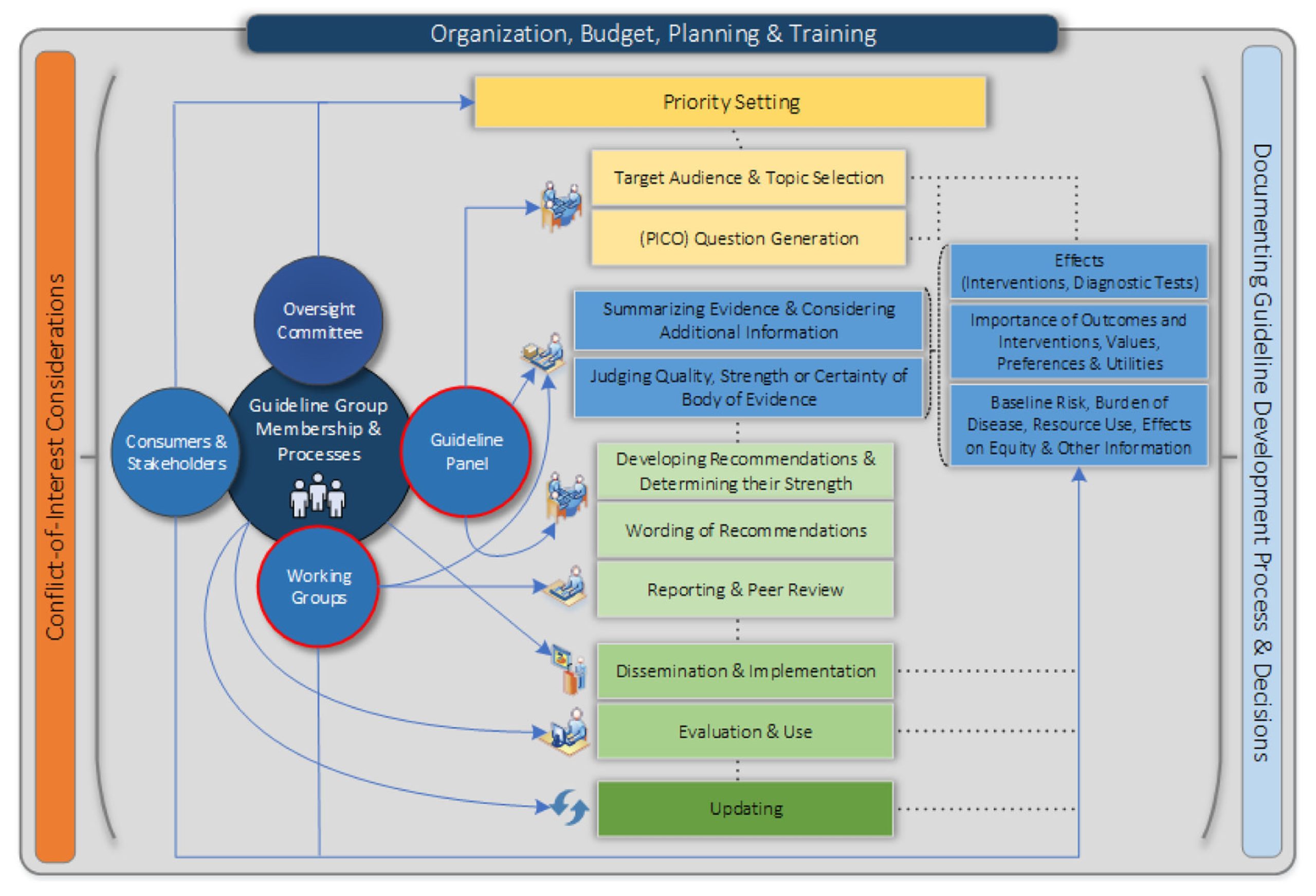
Figure 1: Overview of the guideline development process, as seen from the Guideline International Network (GIN)-McMaster Checklist. Reproduced from Piggott T, Langendam M, Parmelli E, et al. Bringing two worlds closer together: a critical analysis of an integrated approach to guideline development and quality assurance schemes. BMC Health Serv Res. 2021;21(1):172. Published 2021 Feb 24. doi: https://doi.org/10.1186/s12913-020-05819-w under CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).
Guidelines, including those of the AHA/ACC now explicitly attempt to reduce distortions, biases, and competing interests. They aim to minimize this by following a pre-specified, explicit, and transparent set of rules, that apply to all guidelines published by that organization. These guidelines have their foundation as the best available systematic review of the primary evidence. The goal is to use this systematic review and provide recommendations with ratings of the quality of evidence (high, low) along with the strength of recommendation (strong, conditional) [13]. Guidelines in the current age are expected to provide clear descriptions of the relationships between health outcomes and different care options.
Finally, guidelines today are revised regularly as deemed appropriate (by a designated group of individuals) to ensure new evidence gets incorporated in a timely manner [13]. For instance, the 2017 ACC/AHA Guidelines on hypertension [1], received an update through an AHA Scientific Statement published in 2021 on the management of stage 1 hypertension in patients with low ASCVD risk.
In the next part of this series on guidelines, we will continue with what principles guide guideline development.
“The views, opinions, and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness, and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions, or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your health matters. If you think you are having a heart attack, stroke, or another emergency, please call 911 immediately.”