In this AHA session, an international group of physician scientists discussed ways to mitigate immune checkpoint inhibitor (ICI) induced myocarditis and future therapies. The session, moderated by Dr. Sakima Smith MD, MPH, FAHA (from THE Ohio State), and Dr. Doug Tiley highlighted studies by Drs. Burkhard Ludewig, DVM, Dr. Han Zhu, MD, Dr. Alcaide, PhD, Dr. Peter Liu, MD and Dr. Joe-Elie Salem, MD, PhD. The talk began with presenting the problem, basic-science T-cell mechanisms including involvement of microbiota, and ended with a possible targeted therapy, Abatacept. This is a hot topic in the cardio-oncology world considering the high mortality in those affected (up to 50%!) [1].

Source: Cardio-Oncology, Meet Your New Neighbor: Immunology| American Heart
ICIs (eg. ipilimumab, pembrolizumab) are effective targeted therapies in patients with PDL-1/PD-1 expression on tumor cells. Many cancer phenotypes are FDA approved for treatment which includes melanoma, renal cell carcinoma, non-small-cell lung cancer, Hodgkin lymphoma, and more[2]. Although these agents have shown to extend cancer survivorship[3] , they have inadvertent side effects that can lead to myocarditis and cardiomyopathy. ICIs act by “releasing the brake” of T cell immune proliferation. These monoclonal antibodies block PD1/PDL-1 ligands/receptors and allow for T cells to bind to tumor cells leading to reduced tumor burden[3]. Understanding the mechanism for ICI induced myocarditis is partially based on PDL1 knockout mice[4]. Unfortunately, there is cross-reactivity that occurs via binding to cardiac antigens (eg. myosin) leading to the inflammatory response[4].
Dr. Zhu informed us that the risk of this effect includes dual ICI treatment. In addition, early identification is key, considering 50% mortality. Patients may have a drop in their ejection fraction (EF), but have other signs of cardiac injury including brady and tachyarrhythmias. She highlighted that our current data is from FDA sponsored pharmacovigilance databases collected by Dr. Javid Moslehi, who is a pioneer and leading investigator on this subject. A registry created by Dr. Tom Neilan’s lab at Massachusetts General Hospital demonstrated an increased risk of MI and stroke after treatment with ICI[5]. Her group at Stanford along with renowned Dr. Ronald Witteles is using biobanking to identify patients with autoimmune myocarditis and controls to conduct downstream high-throughput immune repertoire analysis. Dr. Alcaide supplemented this talk by adding a novel mechanism. She discussed that reactive oxygen species (ROS) play a role in triggering downstream T cell expansion in the heart. Therefore, there may be a role in anti-oxidant therapy to reduce T cell response. Dr. Liu acknowledged our current pandemic and discussed the added risk of inflammation in the setting of concomitant COVID19 viral infection associated with myocarditis.
During this session, we learned about possible therapies to mitigate myocarditis. Dr. Ludewig discussed his teams work with an ICI mouse model. They explored T cell cross-reactivity that led to the lethality of the disease. There was a heart-gut connection! They found elevation of Bacteroides-specific CD4+ T cells in disease models which suggests that mimic peptides from commensal bacteria can promote inflammatory cardiomyopathy in genetically susceptible patients (those with HLA DQB1*03:01 polymorphisms) by showing increased reactivity against myosin 6 (MYH6) (cardiac antigen). His study suggests that the genetic susceptibility along with cross-reactivity antigens in the heart and potentially the intestine put patients at risk for fulminant myocarditis. Therefore, he proposed the use of antibiotics as a cardioprotective agent by blocking the cross-reactivity that leads to ICI induced myocarditis.
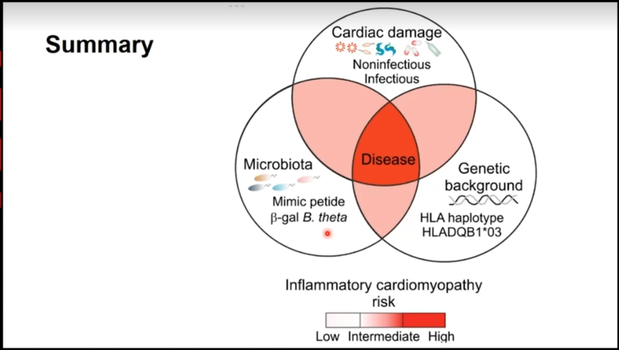
Source: Ludewig: ‘Dangerous gut-heart liaison’| When it comes to matters of the heart, don’t always trust your gut/ Cruz et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 2019; 336, 881-886.
Dr. Joe Elie-Salem (making us jealous by Zooming in from Paris; Ca alors!) ended the session with the introduction of abatacept for therapeutic use in ICI induced myocarditis. Corticosteroids are the mainstay of treatment; however steroid therapy is nonspecific and there are unintended off-target side effects. Specifically, there is a high association with concurrent myasthenia gravis-like syndrome with ICI myocarditis that presents a challenge with the use of steroids. Steroids can lead to an exacerbation of myasthenia crisis which can lead to significant respiratory failure[6]. Based on work with Dr. Moslehi, abatacept (a cytotoxic T-lymphocyte-associated antigen 4 [CTLA-4] agonist, they found that in anti-CTLA4 and Anti—PDL-1 treated disease mouse models, treatment with abatacept reduced myocarditis induced death. This agent will be further explored in a Phase II trial titled: ACHLYS-trial: Phase II trial testing abatacept for ICI-myocarditis.
The take-home points for this session include: 1) ICI used to treat many cancer phenotypes are associated with incident myocarditis with up to 50% mortality 2) Cross-reactivity with cardiac antigens leads to myocyte dysfunction and the clinical sequelae of this includes cardiomyopathy (not always!) and brady/tachyarrhythmias 3) Understanding predisposing immune variants and microbiota (Bacteroides- B. theta) related to immune response associated with this disease is key to identifying all the possible therapies including antibiotics 4) Abatacept is a known T cell immunomodulator and it has a potential role in treating ICI induced myocarditis; especially in those at risk for corticosteroid effects (eg. myasthenia gravis), which will be further explored in a clinical trial.
REFERENCE
- Ball, S., et al., Cardiovascular Toxicities of Immune Checkpoint Inhibitors: JACC Review Topic of the Week. J Am Coll Cardiol, 2019. 74(13): p. 1714-1727.
- Zhou, Y.W., et al., Immune Checkpoint Inhibitor-Associated Cardiotoxicity: Current Understanding on Its Mechanism, Diagnosis and Management. Front Pharmacol, 2019. 10: p. 1350.
- Ferris, R.L., et al., Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med, 2016. 375(19): p. 1856-1867.
- Nishimura, H., et al., Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science, 2001. 291(5502): p. 319-22.
- Drobni, Z.D., et al., Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation, 2020.
- Xing, Q., et al., Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med, 2020. 8(5): p. 250.
“The views, opinions and positions expressed within this blog are those of the author(s) alone and do not represent those of the American Heart Association. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them. The Early Career Voice blog is not intended to provide medical advice or treatment. Only your healthcare provider can provide that. The American Heart Association recommends that you consult your healthcare provider regarding your personal health matters. If you think you are having a heart attack, stroke or another emergency, please call 911 immediately.”