The Lesser Known Vessels in the Heart
The fact needs no reiteration that cardiovascular disease (CVD) remains the leading cause of mortality worldwide – root cause being the obstruction in the coronary arteries, which hinders the blood supply to heart. If this obstruction in the coronary arteries arises in a neonatal rodents or newborn babies, their heart can regenerate with its intrinsic capacity and recover from the disease. However, adults are devoid of such advantage. A newly published study in Cell1 unraveled the mechanism of how the neonatal heart can regenerate while adult cannot. One of the aspects in neonate heart is the development of collateral arteries following the heart injury. While not much is known about these small arteries, they play a significant role in the recovery process by bridging the two conventional arteries or unobstructed areas of a single artery leading to a natural bypass that restores blood flow downstream of an obstruction (Figure 1).
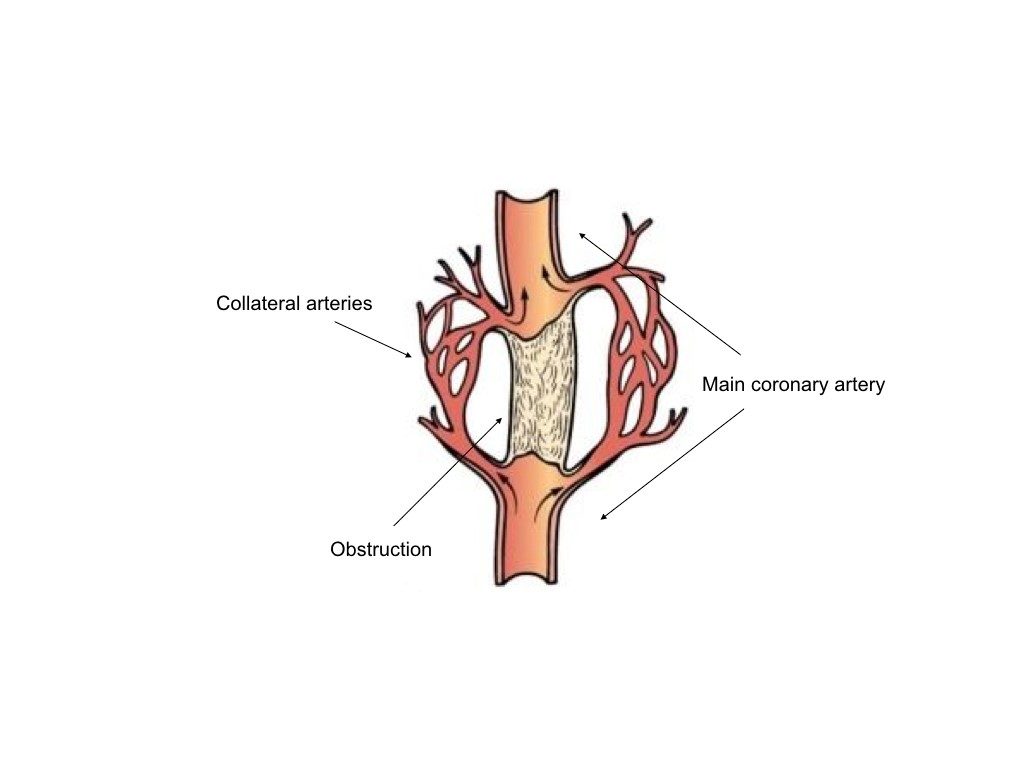
Figure 1: Diagram showing the collateral arteries forming a bypass to restore blood flow below an obstruction.
To see the pattern of these collateral arteries in neonatal vs the adult mice, authors of the study performed a left descending artery (LAD) mimicking a myocardial infarction in P2 mice (when the heart can regenerate) vs P7 (non-regenerative phase of the heart). While they observed significant regeneration of collaterals appearing in the watershed area (midline) of the heart in P2 mice, P7 mice were devoid of these features.
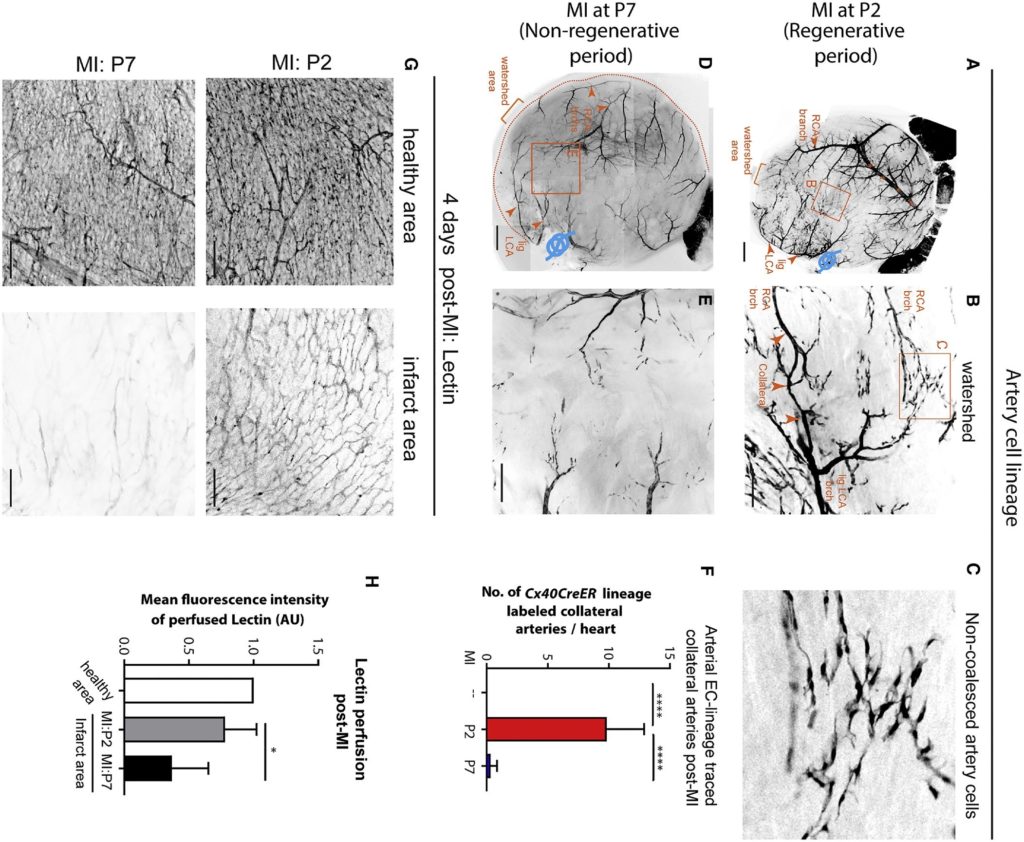
Figure 2: Collateral artery development is restricted to neonatal regenerative phase (source: reference 1).
One thing which dramatically changed in the P2 mice after the injury was the appearance of chemotactic ligand Cxcl12 in the capillary endothelial cells (ECs) which is generally present in arterial ECs in non-injured hearts and plays an important role in guiding coronary EC migration during embryonic development. The necessity of CXCL12 and its receptor CXCR4 in the regeneration process was evident by their deletion from the cells as a result of which cells were unable to form collateral arteries and to recover from the MI injury. The authors in this study made an attempt to explain the model in the following diagram (figure 3).
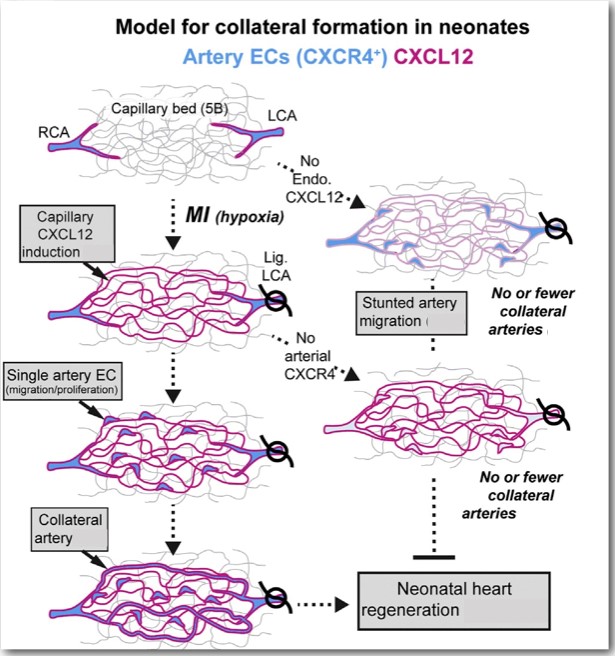
Figure 3: Induction of capillary CXCL12 attracts CXCR4 expressing artery cells out from arteries and into the watershed, where they subsequently proliferate and reassemble into collateral arteries (source: reference 1)
Now, after learning all these exciting concepts, question comes to my mind, what’s its clinical relevance? Does the activation of CXCL12 can somehow stimulate healing in adult heart where endogenous regeneration is limited? To answer this question, the authors injected exogenous CXCL12 directly into the adult mouse heart at the time of MI and to their surprise one dose of CXCL12 stimulated the collateral development process (Video 1). It was previously known that CXCL12 can decrease the scar size and improve heart function after MI2 but now this study suggested that improvement in the functional credentials could have been contributed by formation of collateral arteries in the adult’s injured heart.
Video 1: Single dose of CXCL12 showed an increase in vessel density in the watershed area of heart after MI (source: reference 1)
Thus, this study provided a novel neonatal regenerative pathway which involves migration of arterial endothelial cells to build collateral arteries which can provide blood flow under conditions of infarction or vascular occlusion and has the potential to be harnessed for adult ischemic heart disease (figure 4).
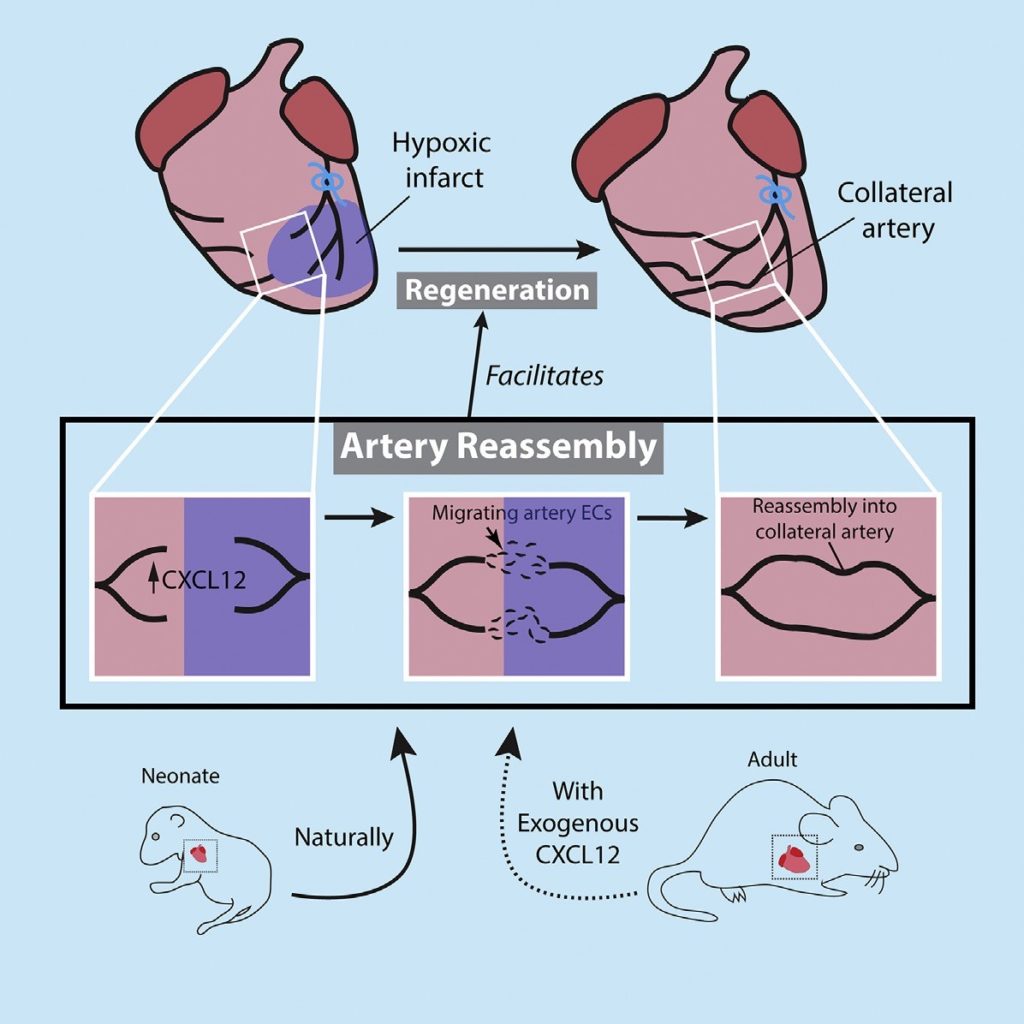
Figure 4: Formation of unique collateral arteries promotes neonate heart regeneration (source: reference 1)
References:
- Das S, Goldstone AB, Wang H, Farry J, D’Amato G, Paulsen MJ, Eskandari A, Hironaka CE, Phansalkar R, Sharma B, Rhee S, Shamskhou EA, Agalliu D, de Jesus Perez V, Woo YJ, Red-Horse K. A UniqueCollateralArtery Development Program Promotes Neonatal Heart Regeneration. Cell. 2019 Feb 21;176(5):1128-1142.e18.
- Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther.2011 Sep;18(9):867-73.
 If you are an early career researcher, this question might have crossed your mind at some point: “What’s the best career choice after finishing my PhD – ‘industry’ or ‘academia?'”
If you are an early career researcher, this question might have crossed your mind at some point: “What’s the best career choice after finishing my PhD – ‘industry’ or ‘academia?'”